Monday Poster Session
Category: Esophagus
P2793 - Esophageal High-Resolution Manometry Can Be Safely and Effectively Performed With Concurrent Glucagon-Like Peptide-1 Receptor Agonist Use
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- KG
Khushboo Gala, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
Khushboo Gala, MD1, Preeyati Chopra, MBBS2, Ashwariya Ohri, MBBS3, Mayank Goyal, MBBS1, George Marek, MD, PhD1, Joseph Murray, MD1, Varan Perananthan, MD1, Don C. Codipilly, MD1, Diana Snyder, MD1, Michael Camilleri, MD, DSc1, Ravi Karthik, MD1
1Mayo Clinic, Rochester, MN; 2Mayo Clinic, Hartford, CT; 3Mayo Clinic, Indianapolis, IN
Introduction: Although commonly performed, esophageal high-resolution manometry (HRM) study completion can be limited by patient tolerance. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are associated with delayed gastric emptying which may impair patients’ ability to complete HRM and has been a concern for aspiration in other intraluminal procedures. We aim to assess the safety and feasibility of performing HRM with concurrent GLP-1RA use.
Methods: Patients who underwent successful and unsuccessful HRM procedures at a tertiary care center from 01/2014-11/2024 were identified. Patients with established gastrointestinal dysmotility, foregut surgery, mechanical obstruction, and opioid use were excluded. Those patients who were actively on a GLP-1 at the time of attempted procedure were classified as “cases”, and the same number of consecutive patients not on a GLP-1RA who attempted the procedure were chosen as “controls”. Continuous variables were reported as mean ± SD and categorical variables as percentages. A 2-sample t-test was used for comparisons between groups.
Results: Among 7194 patients who attempted HRM, 83 patients on GLP-1RA during HRM meeting inclusion criteria (cases) were identified. A total of 74 (89.16%) patients on GLP1RA successfully completed HRM, and 9 (10.84%) could not complete the procedure. In 83 consecutive controls who met inclusion criteria, 77 (92.8%) successfully completed HRM (p = 0.59). Demographics and indications for procedure were similar between groups except for weight and body mass index, which were expectedly higher in cases compared to controls (Table 1A and B). In those patients who had unsuccessful procedures, a similar percentage was completed in cases and controls (15.6% ± 11.8 vs. 14.2% ± 12.4, p = 0.83), and the HRM was continued for similar length of time before aborting (30.6 ± 7.7 minutes vs. 35.8 ± 12.0 minutes, p = 0.37). No patients except one in the control group had a history of intolerance to HRM in the past. Two cases and no controls had a history of nasal septal deviation/repair (p = 0.49). A higher frequency of dysphagia was seen among those controls who had unsuccessful procedures, compared to cases; symptomatology and incidence of anxiety and depression were otherwise similar (Table 2). There were no cases with aspiration or other adverse events.
Discussion: HRM can be safely and effectively completed in patients with GLP-1RA use. Larger, prospective studies are needed to validate these findings.
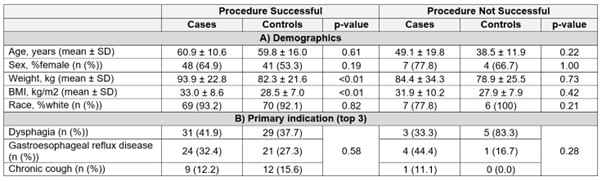
Figure: Table 1: Demographics and Indications for Procedure, By Group
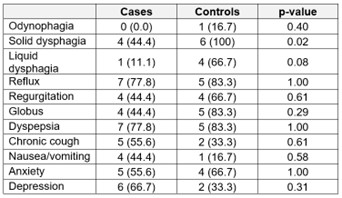
Figure: Table 2: Symptomatology in Unsuccessful Procedures, By Group
Disclosures:
Khushboo Gala indicated no relevant financial relationships.
Preeyati Chopra indicated no relevant financial relationships.
Ashwariya Ohri indicated no relevant financial relationships.
Mayank Goyal indicated no relevant financial relationships.
George Marek indicated no relevant financial relationships.
Joseph Murray indicated no relevant financial relationships.
Varan Perananthan indicated no relevant financial relationships.
Don Codipilly indicated no relevant financial relationships.
Diana Snyder indicated no relevant financial relationships.
Michael Camilleri: Alfasigma – Consultant. Amylyx – Consultant. Biocodex – Grant/Research Support. BioKier – Consultant. Brightseed Bio – Consultant, Grant/Research Support. Coloplast – Consultant. Dignify Therapeutics – Stock Options. Intercept – Consultant. Invea – Consultant. Kallyope – Consultant. McDermott Will & Emery – Consultant. Medpace – Consultant. Monteresearch – Consultant. Neurogastrx – Consultant. NGM Biopharmaceuticals – Grant/Research Support. Pfizer – Grant/Research Support. Phenomix – Stock Options. Renexxion – Consultant. SKYE Bioscience – Consultant. Sumitomo – Consultant. Synlogic – Consultant. Vanda – Grant/Research Support.
Ravi Karthik indicated no relevant financial relationships.
Khushboo Gala, MD1, Preeyati Chopra, MBBS2, Ashwariya Ohri, MBBS3, Mayank Goyal, MBBS1, George Marek, MD, PhD1, Joseph Murray, MD1, Varan Perananthan, MD1, Don C. Codipilly, MD1, Diana Snyder, MD1, Michael Camilleri, MD, DSc1, Ravi Karthik, MD1. P2793 - Esophageal High-Resolution Manometry Can Be Safely and Effectively Performed With Concurrent Glucagon-Like Peptide-1 Receptor Agonist Use, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2Mayo Clinic, Hartford, CT; 3Mayo Clinic, Indianapolis, IN
Introduction: Although commonly performed, esophageal high-resolution manometry (HRM) study completion can be limited by patient tolerance. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are associated with delayed gastric emptying which may impair patients’ ability to complete HRM and has been a concern for aspiration in other intraluminal procedures. We aim to assess the safety and feasibility of performing HRM with concurrent GLP-1RA use.
Methods: Patients who underwent successful and unsuccessful HRM procedures at a tertiary care center from 01/2014-11/2024 were identified. Patients with established gastrointestinal dysmotility, foregut surgery, mechanical obstruction, and opioid use were excluded. Those patients who were actively on a GLP-1 at the time of attempted procedure were classified as “cases”, and the same number of consecutive patients not on a GLP-1RA who attempted the procedure were chosen as “controls”. Continuous variables were reported as mean ± SD and categorical variables as percentages. A 2-sample t-test was used for comparisons between groups.
Results: Among 7194 patients who attempted HRM, 83 patients on GLP-1RA during HRM meeting inclusion criteria (cases) were identified. A total of 74 (89.16%) patients on GLP1RA successfully completed HRM, and 9 (10.84%) could not complete the procedure. In 83 consecutive controls who met inclusion criteria, 77 (92.8%) successfully completed HRM (p = 0.59). Demographics and indications for procedure were similar between groups except for weight and body mass index, which were expectedly higher in cases compared to controls (Table 1A and B). In those patients who had unsuccessful procedures, a similar percentage was completed in cases and controls (15.6% ± 11.8 vs. 14.2% ± 12.4, p = 0.83), and the HRM was continued for similar length of time before aborting (30.6 ± 7.7 minutes vs. 35.8 ± 12.0 minutes, p = 0.37). No patients except one in the control group had a history of intolerance to HRM in the past. Two cases and no controls had a history of nasal septal deviation/repair (p = 0.49). A higher frequency of dysphagia was seen among those controls who had unsuccessful procedures, compared to cases; symptomatology and incidence of anxiety and depression were otherwise similar (Table 2). There were no cases with aspiration or other adverse events.
Discussion: HRM can be safely and effectively completed in patients with GLP-1RA use. Larger, prospective studies are needed to validate these findings.

Figure: Table 1: Demographics and Indications for Procedure, By Group

Figure: Table 2: Symptomatology in Unsuccessful Procedures, By Group
Disclosures:
Khushboo Gala indicated no relevant financial relationships.
Preeyati Chopra indicated no relevant financial relationships.
Ashwariya Ohri indicated no relevant financial relationships.
Mayank Goyal indicated no relevant financial relationships.
George Marek indicated no relevant financial relationships.
Joseph Murray indicated no relevant financial relationships.
Varan Perananthan indicated no relevant financial relationships.
Don Codipilly indicated no relevant financial relationships.
Diana Snyder indicated no relevant financial relationships.
Michael Camilleri: Alfasigma – Consultant. Amylyx – Consultant. Biocodex – Grant/Research Support. BioKier – Consultant. Brightseed Bio – Consultant, Grant/Research Support. Coloplast – Consultant. Dignify Therapeutics – Stock Options. Intercept – Consultant. Invea – Consultant. Kallyope – Consultant. McDermott Will & Emery – Consultant. Medpace – Consultant. Monteresearch – Consultant. Neurogastrx – Consultant. NGM Biopharmaceuticals – Grant/Research Support. Pfizer – Grant/Research Support. Phenomix – Stock Options. Renexxion – Consultant. SKYE Bioscience – Consultant. Sumitomo – Consultant. Synlogic – Consultant. Vanda – Grant/Research Support.
Ravi Karthik indicated no relevant financial relationships.
Khushboo Gala, MD1, Preeyati Chopra, MBBS2, Ashwariya Ohri, MBBS3, Mayank Goyal, MBBS1, George Marek, MD, PhD1, Joseph Murray, MD1, Varan Perananthan, MD1, Don C. Codipilly, MD1, Diana Snyder, MD1, Michael Camilleri, MD, DSc1, Ravi Karthik, MD1. P2793 - Esophageal High-Resolution Manometry Can Be Safely and Effectively Performed With Concurrent Glucagon-Like Peptide-1 Receptor Agonist Use, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
