Monday Poster Session
Category: General Endoscopy
P2975 - Randomized Controlled Trial of Holding Versus Continuing GLP-1 or GIP Agonists Before Upper Endoscopy (OCULUS Trial)
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Akram I. Ahmad, MD (he/him/his)
Cleveland Clinic Florida
Weston, FL
Presenting Author(s)
Award: ACG Outstanding Research Award in the General Endoscopy Category (Trainee)
Award: ACG Presidential Poster Award
Akram I. Ahmad, MD1, Zaid Ansari, MD1, Tasneem Jamal Al-Din, MD1, Ashraf Almomani, MD1, Michael A. Nicolas, BS1, Alaina Miller, BS2, Sara Valencia, MD2, Arjun Chatterjee, MD3, Samita Garg, MD2, Jeffrey Jacobs, MD, MBA4, John Vargo, MD2, Tilak Shah, MD1
1Cleveland Clinic Florida, Weston, FL; 2Cleveland Clinic Foundation, Cleveland, OH; 3Gastroenterology and Hepatology, Cleveland Clinic, Cleveland, OH; 4Cleveland Clinic Florida, Davie, FL
Introduction: Guidelines recommend that patients on daily dosing hold GLP-1 and GIP agonists on the day of endoscopy, and patients on weekly dosing hold the drug a week prior. These recommendations were rapidly incorporated into clinical practice; however, the data supporting them are conflicting and based on low-quality evidence. We hypothesized that holding a single dose of a GLP-1 or GIP agonist prior to endoscopy would be non-inferior to continuing these medications for reducing the risk of clinically relevant residual gastric volume (CR-RGV)
Methods: Single-blinded, randomized controlled trial at two tertiary centers, approved by the Institutional Review Board, and registered on clinicaltrials.gov (NCT06533527). Eligible outpatients on stable maintenance dosing of GLP-1/GIP medications planned for an upper endoscopy (EGD, EUS, or ERCP) were invited to participate (Figure 1). Patients were randomly assigned to either continue GLP-1/GIP agonist therapy (“continue” group) or to hold therapy as outlined in guideline recommendations (“hold” group). Both endoscopy and anesthesiology teams were blinded to study group assignment. Using Farrington-Manning’s test, we estimated that 120 patients were needed (60 in each group) to be 80% certain the upper limit of a one-sided 95% confidence interval would exclude a difference in favor of the standard group of more than 3% (non-inferiority limit). Interim analyses were planned at 20% and 50% of recruitment to assess if the study should be terminated prematurely for safety, or to recalculate the sample size
Results: 60 patients completed the study protocol, and an interim analysis was conducted at 50% enrollment as planned. There were no significant differences in baseline characteristics (Table1). In the intention-to-treat analysis, CR-RGV was significantly higher in the “continue” group (Figure 1). The study was terminated because the Z-value was 2.75, which exceeded the O’Brien-Fleming stopping boundary of 2.5491. There were no cases of CR-RGV in the subgroup who also underwent colonoscopy and were on 1 day of clear liquid diet (0/13 continued, 0/12 held). There were no study-related adverse events
Discussion: Continuing GLP-1 and GIP-1 agonists prior to upper endoscopy significantly increases the risk of CR-RGV that precludes adequate examination. Holding a single dose of the medication significantly reduces the risk of CR-RGV. Clear liquids the day prior to endoscopy may eliminate the risk of CR-RGV, regardless of whether the medication is held or continued
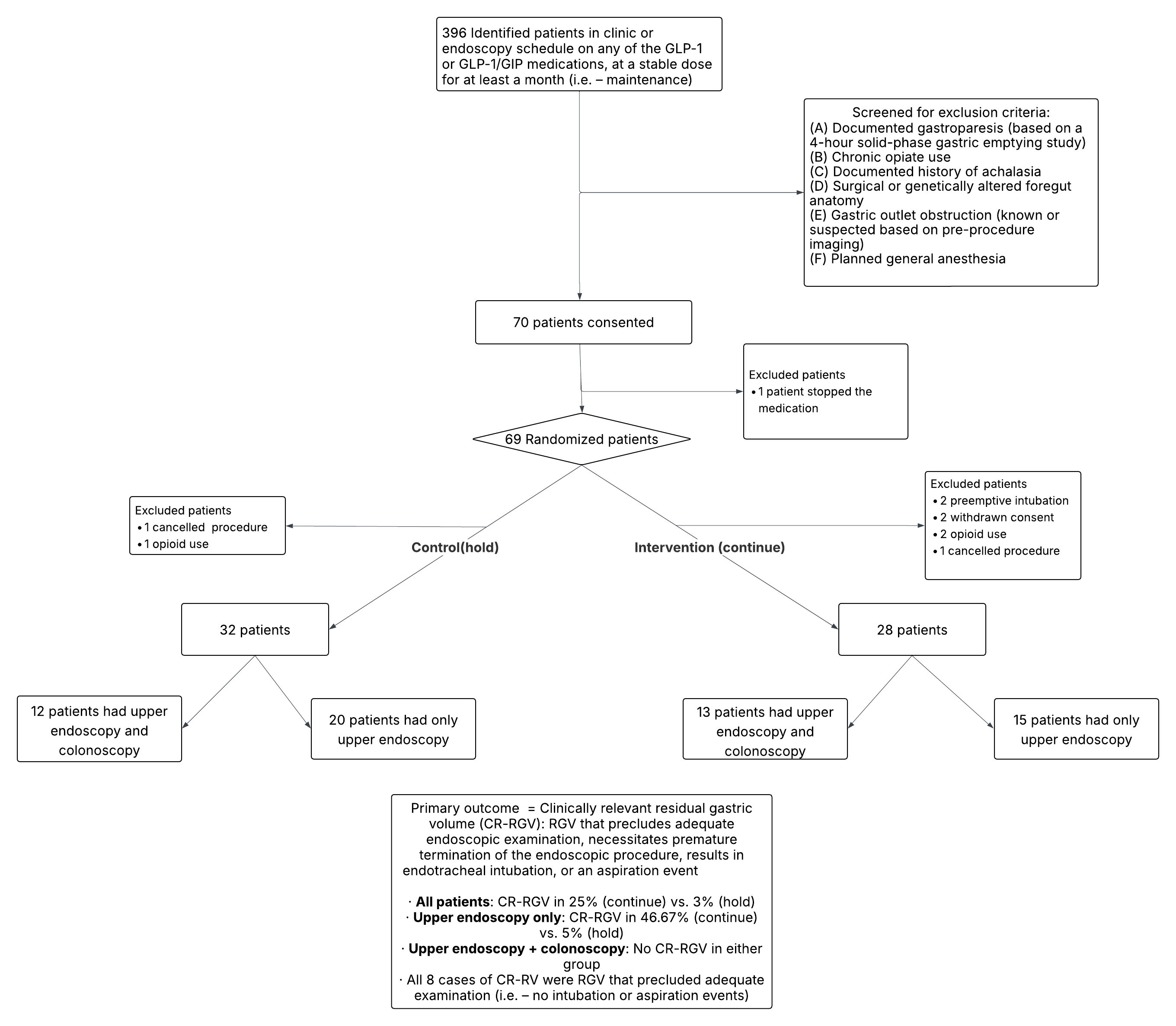
Figure: Figure 1: Study population flowchart
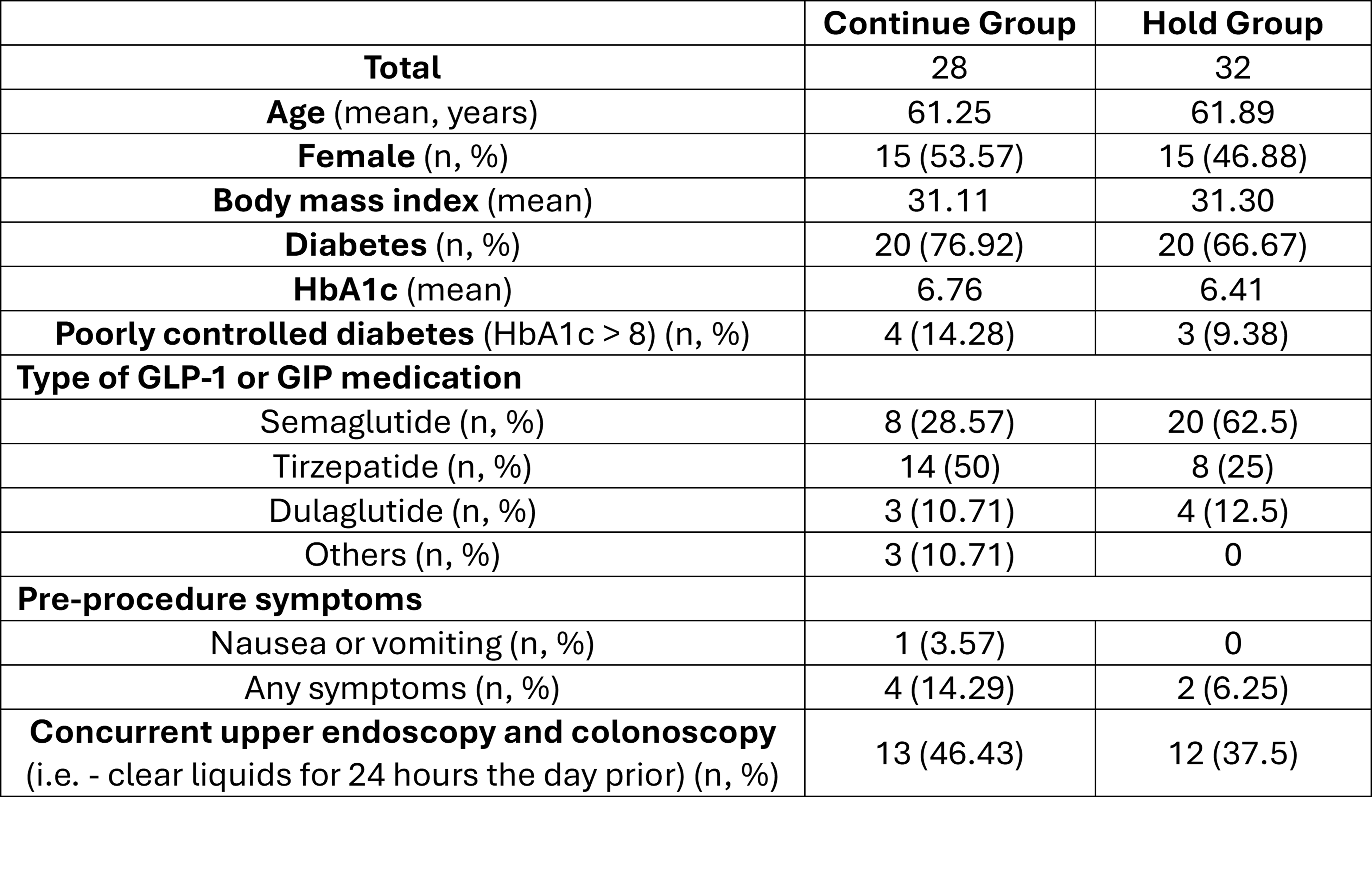
Figure: Table 1: Study population baseline Characteristics
Disclosures:
Akram Ahmad indicated no relevant financial relationships.
Zaid Ansari indicated no relevant financial relationships.
Tasneem Jamal Al-Din indicated no relevant financial relationships.
Ashraf Almomani indicated no relevant financial relationships.
Michael Nicolas indicated no relevant financial relationships.
Alaina Miller indicated no relevant financial relationships.
Sara Valencia indicated no relevant financial relationships.
Arjun Chatterjee indicated no relevant financial relationships.
Samita Garg indicated no relevant financial relationships.
Jeffrey Jacobs indicated no relevant financial relationships.
John Vargo indicated no relevant financial relationships.
Tilak Shah: Steris – Consultant.
Akram I. Ahmad, MD1, Zaid Ansari, MD1, Tasneem Jamal Al-Din, MD1, Ashraf Almomani, MD1, Michael A. Nicolas, BS1, Alaina Miller, BS2, Sara Valencia, MD2, Arjun Chatterjee, MD3, Samita Garg, MD2, Jeffrey Jacobs, MD, MBA4, John Vargo, MD2, Tilak Shah, MD1. P2975 - Randomized Controlled Trial of Holding Versus Continuing GLP-1 or GIP Agonists Before Upper Endoscopy (OCULUS Trial), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Award: ACG Presidential Poster Award
Akram I. Ahmad, MD1, Zaid Ansari, MD1, Tasneem Jamal Al-Din, MD1, Ashraf Almomani, MD1, Michael A. Nicolas, BS1, Alaina Miller, BS2, Sara Valencia, MD2, Arjun Chatterjee, MD3, Samita Garg, MD2, Jeffrey Jacobs, MD, MBA4, John Vargo, MD2, Tilak Shah, MD1
1Cleveland Clinic Florida, Weston, FL; 2Cleveland Clinic Foundation, Cleveland, OH; 3Gastroenterology and Hepatology, Cleveland Clinic, Cleveland, OH; 4Cleveland Clinic Florida, Davie, FL
Introduction: Guidelines recommend that patients on daily dosing hold GLP-1 and GIP agonists on the day of endoscopy, and patients on weekly dosing hold the drug a week prior. These recommendations were rapidly incorporated into clinical practice; however, the data supporting them are conflicting and based on low-quality evidence. We hypothesized that holding a single dose of a GLP-1 or GIP agonist prior to endoscopy would be non-inferior to continuing these medications for reducing the risk of clinically relevant residual gastric volume (CR-RGV)
Methods: Single-blinded, randomized controlled trial at two tertiary centers, approved by the Institutional Review Board, and registered on clinicaltrials.gov (NCT06533527). Eligible outpatients on stable maintenance dosing of GLP-1/GIP medications planned for an upper endoscopy (EGD, EUS, or ERCP) were invited to participate (Figure 1). Patients were randomly assigned to either continue GLP-1/GIP agonist therapy (“continue” group) or to hold therapy as outlined in guideline recommendations (“hold” group). Both endoscopy and anesthesiology teams were blinded to study group assignment. Using Farrington-Manning’s test, we estimated that 120 patients were needed (60 in each group) to be 80% certain the upper limit of a one-sided 95% confidence interval would exclude a difference in favor of the standard group of more than 3% (non-inferiority limit). Interim analyses were planned at 20% and 50% of recruitment to assess if the study should be terminated prematurely for safety, or to recalculate the sample size
Results: 60 patients completed the study protocol, and an interim analysis was conducted at 50% enrollment as planned. There were no significant differences in baseline characteristics (Table1). In the intention-to-treat analysis, CR-RGV was significantly higher in the “continue” group (Figure 1). The study was terminated because the Z-value was 2.75, which exceeded the O’Brien-Fleming stopping boundary of 2.5491. There were no cases of CR-RGV in the subgroup who also underwent colonoscopy and were on 1 day of clear liquid diet (0/13 continued, 0/12 held). There were no study-related adverse events
Discussion: Continuing GLP-1 and GIP-1 agonists prior to upper endoscopy significantly increases the risk of CR-RGV that precludes adequate examination. Holding a single dose of the medication significantly reduces the risk of CR-RGV. Clear liquids the day prior to endoscopy may eliminate the risk of CR-RGV, regardless of whether the medication is held or continued

Figure: Figure 1: Study population flowchart

Figure: Table 1: Study population baseline Characteristics
Disclosures:
Akram Ahmad indicated no relevant financial relationships.
Zaid Ansari indicated no relevant financial relationships.
Tasneem Jamal Al-Din indicated no relevant financial relationships.
Ashraf Almomani indicated no relevant financial relationships.
Michael Nicolas indicated no relevant financial relationships.
Alaina Miller indicated no relevant financial relationships.
Sara Valencia indicated no relevant financial relationships.
Arjun Chatterjee indicated no relevant financial relationships.
Samita Garg indicated no relevant financial relationships.
Jeffrey Jacobs indicated no relevant financial relationships.
John Vargo indicated no relevant financial relationships.
Tilak Shah: Steris – Consultant.
Akram I. Ahmad, MD1, Zaid Ansari, MD1, Tasneem Jamal Al-Din, MD1, Ashraf Almomani, MD1, Michael A. Nicolas, BS1, Alaina Miller, BS2, Sara Valencia, MD2, Arjun Chatterjee, MD3, Samita Garg, MD2, Jeffrey Jacobs, MD, MBA4, John Vargo, MD2, Tilak Shah, MD1. P2975 - Randomized Controlled Trial of Holding Versus Continuing GLP-1 or GIP Agonists Before Upper Endoscopy (OCULUS Trial), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

