Monday Poster Session
Category: General Endoscopy
P2972 - Colonoscopy Compression Device Significantly Reduces Ergonomic Risk for Endoscopy Staff: A Multi-Center Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Lauren Shea, MD
St. Peter's Health
Helena, MT
Presenting Author(s)
Award: ACG Outstanding Research Award in the General Endoscopy Category
Award: ACG Presidential Poster Award
Lauren Shea, MD1, Divya Ashat, MBBS2, Anthony Beisler, MD3, Danielle DeLeeuw, 4, Jeffrey Goldman, MD, MBA2, David Gutman, MD5, Noufal Jajeh, MD3, Jill Lee, 5, Rick Norton, 5, Timothy Peterson, MD4, Brandon Scott, DO5, Tamara James, MA6
1St. Peter's Health, Helena, MT; 2University of Iowa Hospitals & Clinics, Iowa City, IA; 3Mary Rutan Health, Bellefontaine, OH; 4Meeker Memorial Hospital & Clinics, Litchfield, MN; 5Portneuf Medical Center, Portneuf, ID; 6Duke University Health System, Durham, NC
Introduction: Colonoscopy procedures often expose endoscopy staff to ergonomic risks, such as musculoskeletal (MSK) disorders, due to manual abdominal pressure and frequent patient repositioning. ColoWrap, an external compression device designed to provide adjustable abdominal pressure and mitigate looping during colonoscopies, reduces the need for manual staff interventions. Previous studies have shown that using ColoWrap decreases the frequency of nurse-reported MSK pain. This study aimed to quantify ergonomic risks during colonoscopies performed with and without the ColoWrap device, using the validated Colonoscopy Staff Strain Index (CSSI).
Methods: This multi-site study analyzed 388 colonoscopy procedures performed by eight endoscopists across six hospital sites. Data were collected over two periods: one month prior to ColoWrap adoption (297 procedures, baseline group) and during its use (91 procedures, ColoWrap group). The analysis included only endoscopists who performed at least five procedures in both groups. Procedures where ColoWrap was applied mid-procedure were excluded. CSSI scores, calculated from manual abdominal pressure and patient repositioning metrics, were used to assess ergonomic risk. Scores above 1.0 are considered to indicate increased risk of staff ergonomic injury. Patient populations were similar between groups, except for BMI, reflecting physician preference for using ColoWrap in patients with higher BMI.
Results: The mean CSSI score significantly decreased from 2.4 ± 4.3 in the baseline group to 1.0 ± 2.6 in the ColoWrap group (p = 0.0003). Procedures with excessive ergonomic risk (CSSI > 5) were less frequent in the ColoWrap group (7.8% vs. 17.0%, p = 0.02). ColoWrap also reduced the need for manual abdominal pressure (16.4% vs. 42.1%, p < 0.0001) and decreased patient repositioning (1.1% vs. 7.7%, p = 0.007). These findings highlight the significant ergonomic benefits of using ColoWrap, even in cases likely perceived as more challenging.
Discussion: The ColoWrap device significantly reduces ergonomic risks associated with colonoscopy procedures, as demonstrated by lower CSSI scores, fewer high-risk procedures, reduced manual abdominal pressure, and minimal patient repositioning. These compelling results underscore ColoWrap’s potential to enhance staff safety, even in procedures anticipated to be more difficult. Future research should explore its long-term effects on reducing musculoskeletal injuries among endoscopy staff.
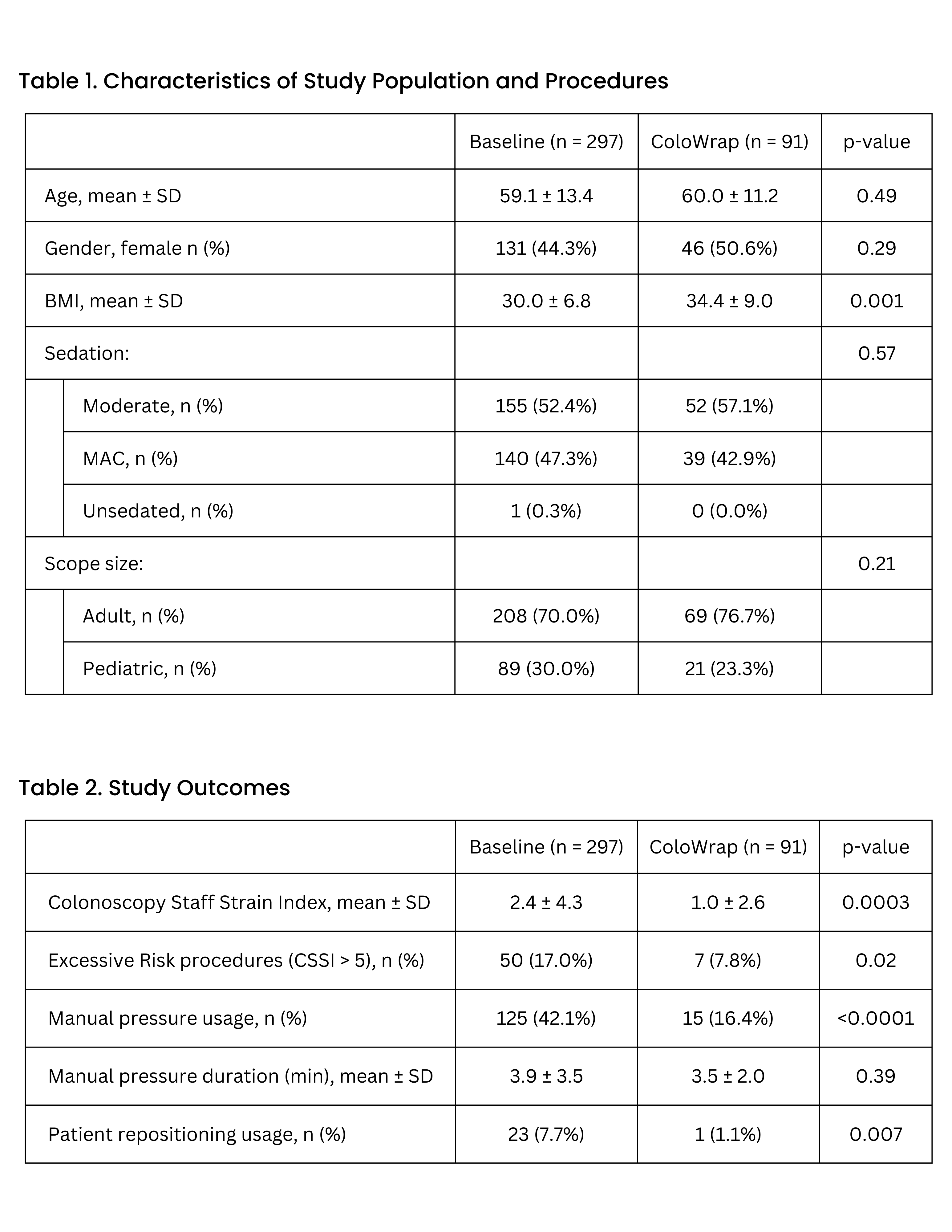
Figure: Table 1. Population and Procedure Characteristics; Table 2. Study Outcomes
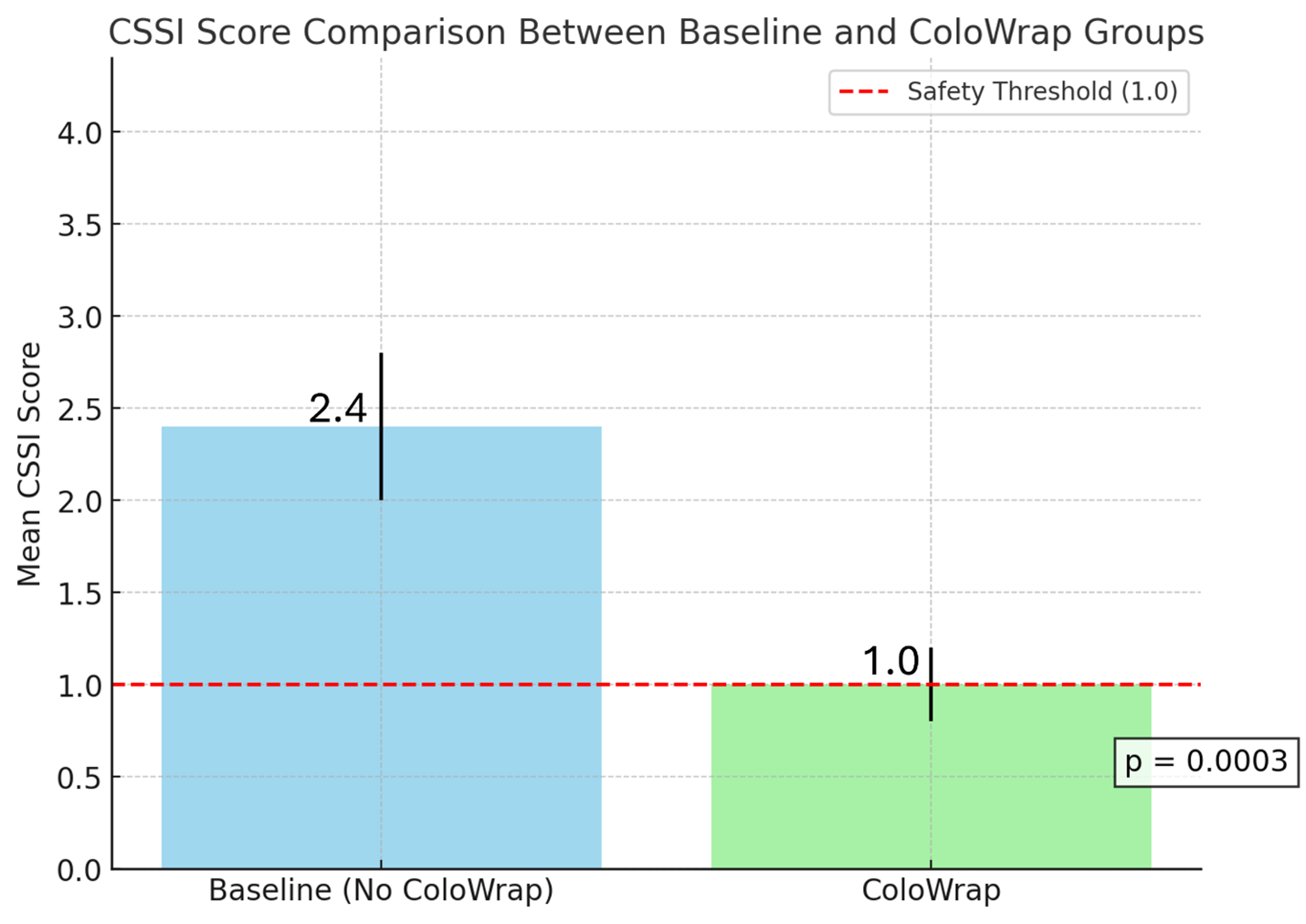
Figure: Comparison of Staff Colonoscopy Ergonomic Risk Scores (CSSI) between colonoscopies performed with and without ColoWrap device.
Disclosures:
Lauren Shea indicated no relevant financial relationships.
Divya Ashat indicated no relevant financial relationships.
Anthony Beisler indicated no relevant financial relationships.
Danielle DeLeeuw indicated no relevant financial relationships.
Jeffrey Goldman indicated no relevant financial relationships.
David Gutman indicated no relevant financial relationships.
Noufal Jajeh indicated no relevant financial relationships.
Jill Lee indicated no relevant financial relationships.
Rick Norton indicated no relevant financial relationships.
Timothy Peterson indicated no relevant financial relationships.
Brandon Scott indicated no relevant financial relationships.
Tamara James: ColoWrap, Inc. – Consultant.
Lauren Shea, MD1, Divya Ashat, MBBS2, Anthony Beisler, MD3, Danielle DeLeeuw, 4, Jeffrey Goldman, MD, MBA2, David Gutman, MD5, Noufal Jajeh, MD3, Jill Lee, 5, Rick Norton, 5, Timothy Peterson, MD4, Brandon Scott, DO5, Tamara James, MA6. P2972 - Colonoscopy Compression Device Significantly Reduces Ergonomic Risk for Endoscopy Staff: A Multi-Center Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Award: ACG Presidential Poster Award
Lauren Shea, MD1, Divya Ashat, MBBS2, Anthony Beisler, MD3, Danielle DeLeeuw, 4, Jeffrey Goldman, MD, MBA2, David Gutman, MD5, Noufal Jajeh, MD3, Jill Lee, 5, Rick Norton, 5, Timothy Peterson, MD4, Brandon Scott, DO5, Tamara James, MA6
1St. Peter's Health, Helena, MT; 2University of Iowa Hospitals & Clinics, Iowa City, IA; 3Mary Rutan Health, Bellefontaine, OH; 4Meeker Memorial Hospital & Clinics, Litchfield, MN; 5Portneuf Medical Center, Portneuf, ID; 6Duke University Health System, Durham, NC
Introduction: Colonoscopy procedures often expose endoscopy staff to ergonomic risks, such as musculoskeletal (MSK) disorders, due to manual abdominal pressure and frequent patient repositioning. ColoWrap, an external compression device designed to provide adjustable abdominal pressure and mitigate looping during colonoscopies, reduces the need for manual staff interventions. Previous studies have shown that using ColoWrap decreases the frequency of nurse-reported MSK pain. This study aimed to quantify ergonomic risks during colonoscopies performed with and without the ColoWrap device, using the validated Colonoscopy Staff Strain Index (CSSI).
Methods: This multi-site study analyzed 388 colonoscopy procedures performed by eight endoscopists across six hospital sites. Data were collected over two periods: one month prior to ColoWrap adoption (297 procedures, baseline group) and during its use (91 procedures, ColoWrap group). The analysis included only endoscopists who performed at least five procedures in both groups. Procedures where ColoWrap was applied mid-procedure were excluded. CSSI scores, calculated from manual abdominal pressure and patient repositioning metrics, were used to assess ergonomic risk. Scores above 1.0 are considered to indicate increased risk of staff ergonomic injury. Patient populations were similar between groups, except for BMI, reflecting physician preference for using ColoWrap in patients with higher BMI.
Results: The mean CSSI score significantly decreased from 2.4 ± 4.3 in the baseline group to 1.0 ± 2.6 in the ColoWrap group (p = 0.0003). Procedures with excessive ergonomic risk (CSSI > 5) were less frequent in the ColoWrap group (7.8% vs. 17.0%, p = 0.02). ColoWrap also reduced the need for manual abdominal pressure (16.4% vs. 42.1%, p < 0.0001) and decreased patient repositioning (1.1% vs. 7.7%, p = 0.007). These findings highlight the significant ergonomic benefits of using ColoWrap, even in cases likely perceived as more challenging.
Discussion: The ColoWrap device significantly reduces ergonomic risks associated with colonoscopy procedures, as demonstrated by lower CSSI scores, fewer high-risk procedures, reduced manual abdominal pressure, and minimal patient repositioning. These compelling results underscore ColoWrap’s potential to enhance staff safety, even in procedures anticipated to be more difficult. Future research should explore its long-term effects on reducing musculoskeletal injuries among endoscopy staff.

Figure: Table 1. Population and Procedure Characteristics; Table 2. Study Outcomes

Figure: Comparison of Staff Colonoscopy Ergonomic Risk Scores (CSSI) between colonoscopies performed with and without ColoWrap device.
Disclosures:
Lauren Shea indicated no relevant financial relationships.
Divya Ashat indicated no relevant financial relationships.
Anthony Beisler indicated no relevant financial relationships.
Danielle DeLeeuw indicated no relevant financial relationships.
Jeffrey Goldman indicated no relevant financial relationships.
David Gutman indicated no relevant financial relationships.
Noufal Jajeh indicated no relevant financial relationships.
Jill Lee indicated no relevant financial relationships.
Rick Norton indicated no relevant financial relationships.
Timothy Peterson indicated no relevant financial relationships.
Brandon Scott indicated no relevant financial relationships.
Tamara James: ColoWrap, Inc. – Consultant.
Lauren Shea, MD1, Divya Ashat, MBBS2, Anthony Beisler, MD3, Danielle DeLeeuw, 4, Jeffrey Goldman, MD, MBA2, David Gutman, MD5, Noufal Jajeh, MD3, Jill Lee, 5, Rick Norton, 5, Timothy Peterson, MD4, Brandon Scott, DO5, Tamara James, MA6. P2972 - Colonoscopy Compression Device Significantly Reduces Ergonomic Risk for Endoscopy Staff: A Multi-Center Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.


