Monday Poster Session
Category: IBD
P3219 - Optimal Screening Intervals for Dysplasia in Inflammatory Bowel Disease: Results of a Markov Model
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Kate Lee, MD, MS
Duke University Medical Center
Philadelphia, PA
Presenting Author(s)
Award: ACG Presidential Poster Award
Kate L. Karlin, MD, MS1, Alice A. Agyekum, BS2, Jane E. Onken, MD, MHS1, Bo Shen, MD2, Francesca Lim, MS2, Jordan Axelrad, MD, MPH3, Aasma Shaukat, MD, MPH, FACG4, Chin Hur, MD, MPH2, Adam Faye, MD, MS5
1Duke University Medical Center, Durham, NC; 2Columbia University Irving Medical Center, New York, NY; 3Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine, New York, NY; 4NYU Grossman School of Medicine, Division of Gastroenterology and Hepatology, New York, NY; 5NYU Langone Health, New York, NY
Introduction: Current guidelines recommend frequent colorectal cancer (CRC) screening in patients with inflammatory bowel disease (IBD), but the optimal screening interval remains unclear. We aimed to model dysplasia development in IBD to assess the cost-effectiveness and clinical impact of various CRC screening intervals.
Methods: We developed a Markov model in TreeAge Pro 2024 using a base case of a 30-year-old undergoing their first surveillance colonoscopy 8 years after IBD diagnosis. Initial health states of no dysplasia, low grade dysplasia, high grade dysplasia, and CRC were based on recently published prevalence estimates. We compared three strategies: (1) a natural history arm with only the initial surveillance colonoscopy, (2) surveillance colonoscopy every 2 years for patients without dysplasia, and (3) an alternative strategy with colonoscopy every 4 years for patients without dysplasia. In all arms, patients could progress to advanced stages of dysplasia or CRC, with follow-up until death or age 100. The model was calibrated to CRC incidence and mortality using DevCan (SEER 2000-2020) and adjusted for IBD using a relative risk from the literature. Deterministic and probabilistic sensitivity analyses included variations in colonoscopy adherence.
Results: The cost-effective strategy was continuing with a 4-year surveillance interval for patients without dysplasia. This strategy yielded an incremental cost-effectiveness ratio (ICER) of $6,043 per quality-adjusted life year (QALY), while the 2-year strategy had an ICER of $171,615 per QALY (Table). CRC incidence was slightly higher with the 4-year strategy (4.5% vs. 3.8%), with minimal differences in local-stage detection (59% vs. 60%) and CRC-related mortality (0.18% vs. 0.15%). The 4-year strategy remained cost-effective across sensitivity analyses, with dysplasia detection rates and colonoscopy cost as key drivers. Probabilistic analysis favored the 4-year approach in 70% of simulations (Figure). Regarding resource utilization, colonoscopy use per 100 persons screened was 1,283 for the 4-year strategy vs. 1,719 for the 2-year strategy, and only 17 in the natural history arm.
Discussion: Our model shows that extending surveillance colonoscopy to every 4 years in IBD patients without dysplasia is the cost-effective strategy. These findings support a more efficient approach to dysplasia surveillance that preserves patient safety while optimizing healthcare resources.
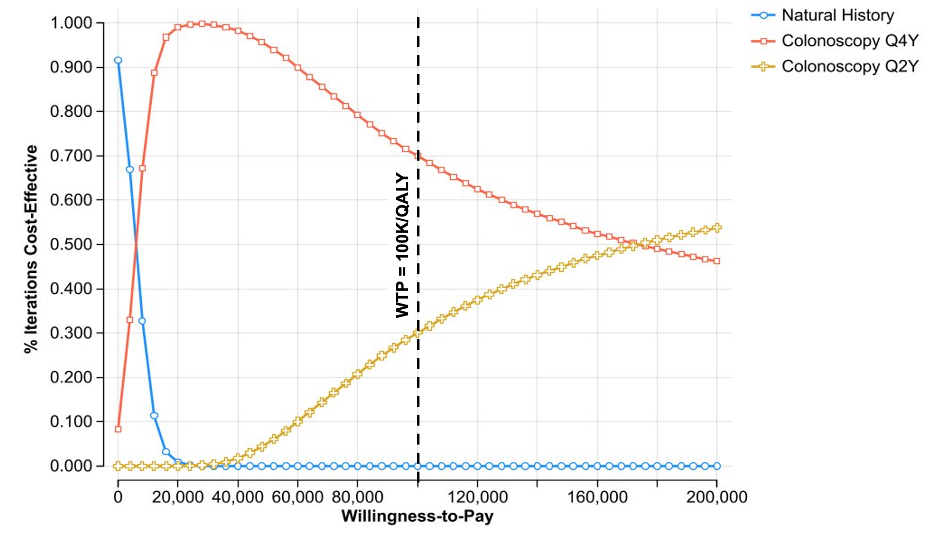
Figure: Table. Cost-Effectiveness Analysis Results.
*persons screened via colonoscopy
Abbreviations: Abs. (absolute), Colo (colonoscopy), CRC (colorectal cancer), HGD (high grade dysplasia), ICER (incremental cost-effectiveness ratio), LGD (low grade dysplasia), NH (natural history), QALY (quality-adjusted life year), ref (reference)
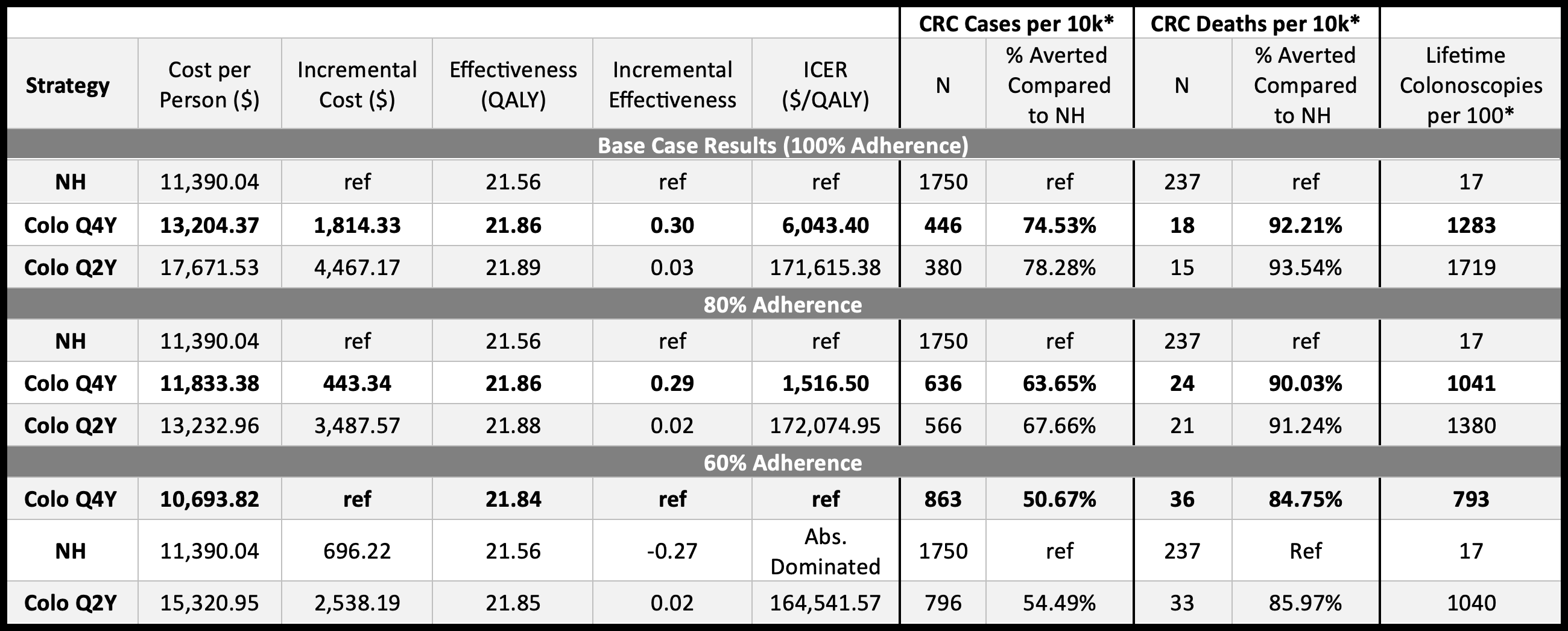
Figure: Figure. Cost-Effectiveness Acceptability Curve.
Abbreviations: QALY (quality-adjusted life year), WTP (willingness to pay)
Disclosures:
Kate Karlin: American College of Gastroenterology – Grant/Research Support.
Alice Agyekum indicated no relevant financial relationships.
Jane Onken: Merck – Stock-publicly held company(excluding mutual/index funds).
Bo Shen: Abbvie – Grant/Research Support. GIE Medical – Grant/Research Support. Janssen – Consultant, Grant/Research Support. Takeda – Grant/Research Support.
Francesca Lim indicated no relevant financial relationships.
Jordan Axelrad: Abbvie – Advisory Committee/Board Member, Consultant, Honorarium. Abivax – Advisory Committee/Board Member, Consultant, Honorarium. Adiso – Advisory Committee/Board Member, Consultant, Honorarium. BioFire Diagnostics – Grant/Research Support. Biomerieux – Advisory Committee/Board Member, Consultant, Honorarium. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant, Honorarium. Celltrion – Advisory Committee/Board Member, Consultant, Honorarium. Ferring – Advisory Committee/Board Member, Consultant, Honorarium. Fresenius – Advisory Committee/Board Member, Consultant, Honorarium. Genentech – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Janssen – Grant/Research Support. Janssen – Honorarium. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant, Honorarium. NIH NIDDK Diseases K23DK124570 – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. The Crohn's and Colitis Foundation (#878246) – Grant/Research Support. The Judith & Stewart Colton Center for Autoimmunity – Grant/Research Support. Vedanta – Advisory Committee/Board Member, Consultant, Honorarium.
Aasma Shaukat: Freenome inc – Consultant.
Chin Hur: Cylinder Health – Advisor or Review Panel Member. Guardant Health – Consultant. Value Health Analytics – Consultant.
Adam Faye: AbbVie – Honorarium. Eli Lilly – Consultant. NIH Grant K76AG083286 – Grant/Research Support. Takeda – Honorarium. The American College of Gastroenterology – Grant/Research Support. The Crohn's and Colitis Foundation – Grant/Research Support.
Kate L. Karlin, MD, MS1, Alice A. Agyekum, BS2, Jane E. Onken, MD, MHS1, Bo Shen, MD2, Francesca Lim, MS2, Jordan Axelrad, MD, MPH3, Aasma Shaukat, MD, MPH, FACG4, Chin Hur, MD, MPH2, Adam Faye, MD, MS5. P3219 - Optimal Screening Intervals for Dysplasia in Inflammatory Bowel Disease: Results of a Markov Model, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Kate L. Karlin, MD, MS1, Alice A. Agyekum, BS2, Jane E. Onken, MD, MHS1, Bo Shen, MD2, Francesca Lim, MS2, Jordan Axelrad, MD, MPH3, Aasma Shaukat, MD, MPH, FACG4, Chin Hur, MD, MPH2, Adam Faye, MD, MS5
1Duke University Medical Center, Durham, NC; 2Columbia University Irving Medical Center, New York, NY; 3Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine, New York, NY; 4NYU Grossman School of Medicine, Division of Gastroenterology and Hepatology, New York, NY; 5NYU Langone Health, New York, NY
Introduction: Current guidelines recommend frequent colorectal cancer (CRC) screening in patients with inflammatory bowel disease (IBD), but the optimal screening interval remains unclear. We aimed to model dysplasia development in IBD to assess the cost-effectiveness and clinical impact of various CRC screening intervals.
Methods: We developed a Markov model in TreeAge Pro 2024 using a base case of a 30-year-old undergoing their first surveillance colonoscopy 8 years after IBD diagnosis. Initial health states of no dysplasia, low grade dysplasia, high grade dysplasia, and CRC were based on recently published prevalence estimates. We compared three strategies: (1) a natural history arm with only the initial surveillance colonoscopy, (2) surveillance colonoscopy every 2 years for patients without dysplasia, and (3) an alternative strategy with colonoscopy every 4 years for patients without dysplasia. In all arms, patients could progress to advanced stages of dysplasia or CRC, with follow-up until death or age 100. The model was calibrated to CRC incidence and mortality using DevCan (SEER 2000-2020) and adjusted for IBD using a relative risk from the literature. Deterministic and probabilistic sensitivity analyses included variations in colonoscopy adherence.
Results: The cost-effective strategy was continuing with a 4-year surveillance interval for patients without dysplasia. This strategy yielded an incremental cost-effectiveness ratio (ICER) of $6,043 per quality-adjusted life year (QALY), while the 2-year strategy had an ICER of $171,615 per QALY (Table). CRC incidence was slightly higher with the 4-year strategy (4.5% vs. 3.8%), with minimal differences in local-stage detection (59% vs. 60%) and CRC-related mortality (0.18% vs. 0.15%). The 4-year strategy remained cost-effective across sensitivity analyses, with dysplasia detection rates and colonoscopy cost as key drivers. Probabilistic analysis favored the 4-year approach in 70% of simulations (Figure). Regarding resource utilization, colonoscopy use per 100 persons screened was 1,283 for the 4-year strategy vs. 1,719 for the 2-year strategy, and only 17 in the natural history arm.
Discussion: Our model shows that extending surveillance colonoscopy to every 4 years in IBD patients without dysplasia is the cost-effective strategy. These findings support a more efficient approach to dysplasia surveillance that preserves patient safety while optimizing healthcare resources.

Figure: Table. Cost-Effectiveness Analysis Results.
*persons screened via colonoscopy
Abbreviations: Abs. (absolute), Colo (colonoscopy), CRC (colorectal cancer), HGD (high grade dysplasia), ICER (incremental cost-effectiveness ratio), LGD (low grade dysplasia), NH (natural history), QALY (quality-adjusted life year), ref (reference)

Figure: Figure. Cost-Effectiveness Acceptability Curve.
Abbreviations: QALY (quality-adjusted life year), WTP (willingness to pay)
Disclosures:
Kate Karlin: American College of Gastroenterology – Grant/Research Support.
Alice Agyekum indicated no relevant financial relationships.
Jane Onken: Merck – Stock-publicly held company(excluding mutual/index funds).
Bo Shen: Abbvie – Grant/Research Support. GIE Medical – Grant/Research Support. Janssen – Consultant, Grant/Research Support. Takeda – Grant/Research Support.
Francesca Lim indicated no relevant financial relationships.
Jordan Axelrad: Abbvie – Advisory Committee/Board Member, Consultant, Honorarium. Abivax – Advisory Committee/Board Member, Consultant, Honorarium. Adiso – Advisory Committee/Board Member, Consultant, Honorarium. BioFire Diagnostics – Grant/Research Support. Biomerieux – Advisory Committee/Board Member, Consultant, Honorarium. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant, Honorarium. Celltrion – Advisory Committee/Board Member, Consultant, Honorarium. Ferring – Advisory Committee/Board Member, Consultant, Honorarium. Fresenius – Advisory Committee/Board Member, Consultant, Honorarium. Genentech – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Janssen – Grant/Research Support. Janssen – Honorarium. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant, Honorarium. NIH NIDDK Diseases K23DK124570 – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. The Crohn's and Colitis Foundation (#878246) – Grant/Research Support. The Judith & Stewart Colton Center for Autoimmunity – Grant/Research Support. Vedanta – Advisory Committee/Board Member, Consultant, Honorarium.
Aasma Shaukat: Freenome inc – Consultant.
Chin Hur: Cylinder Health – Advisor or Review Panel Member. Guardant Health – Consultant. Value Health Analytics – Consultant.
Adam Faye: AbbVie – Honorarium. Eli Lilly – Consultant. NIH Grant K76AG083286 – Grant/Research Support. Takeda – Honorarium. The American College of Gastroenterology – Grant/Research Support. The Crohn's and Colitis Foundation – Grant/Research Support.
Kate L. Karlin, MD, MS1, Alice A. Agyekum, BS2, Jane E. Onken, MD, MHS1, Bo Shen, MD2, Francesca Lim, MS2, Jordan Axelrad, MD, MPH3, Aasma Shaukat, MD, MPH, FACG4, Chin Hur, MD, MPH2, Adam Faye, MD, MS5. P3219 - Optimal Screening Intervals for Dysplasia in Inflammatory Bowel Disease: Results of a Markov Model, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

