Monday Poster Session
Category: IBD
P3208 - Assessment of Serious Adverse Events in Patients Treated With Advanced Inflammatory Bowel Disease Therapies in a Real-World Cohort
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- RS
Ritu Singh, MD (he/him/his)
University of Illinois College of Medicine
Peoria, IL
Presenting Author(s)
Ritu Singh, MD1, Muhammad Sheharyar Warraich, MD2, Hasan Shoaib, MD1, Nikhil Kalva, MD1
1University of Illinois College of Medicine, Peoria, IL; 2University of Illinois, Peoria, IL
Introduction: There have been phenomenal advancements in the treatment of moderate to severe inflammatory bowel diseases (IBD) over the last decade with improvement in disease remission rates and reduction in complications. However, use of advanced IBD therapies is associated with risk of adverse events ranging from opportunistic infections to solid and hematologic malignancies. There is limited real-world data on the safety of advanced IBD therapies. We aim to explore the real-world data on the serious adverse events associated with the use of advanced therapies.
Methods: We performed a retrospective cohort study utilizing a large research network (TriNetX). Adults with IBD from January 2011 to December 2023 were identified using ICD-10-CM codes for Crohn’s disease and ulcerative colitis. Advanced IBD therapies (TNF inhibitors, integrin inhibitors, interleukin inhibitors, JAK inhibitors) and thiopurines (azathioprine, 6-mercaptoprine) were identified using RxNorm codes. The primary outcome was the rate of opportunistic infections, acute hepatitis B or flare of acute hepatitis B, hematologic malignancies, all skin cancers and non-melanoma skin cancer, and all solid malignancies in patients with IBD treated with thiopurines and individual advanced IBD therapies compared to none of the above therapies.
Results: We identified 570,103 patients with IBD (median follow 1095 days). Among all IBD patients, 89,716 (15.74%) patients were treated with TNF inhibitors, 28,712 (5.04%) with IL-inhibitors, 24,095 with thiopurines (4.23%), 23,980 (4.21%) with integrin inhibitors and 7,627 (1.34%) were treated with JAK inhibitors. The risk of opportunistic infections was highest in patients on JAK inhibitors (RR 3.14 [95% CI 2.34-4.22]) and thiopurines (risk ratio, RR 2.26 [95% confidence interval, CI 1.93-2.65]). Thiopurine use was associated with the highest risk of all skin cancers (RR 1.77 [95% CI 1.57-1.99] and non-melanoma (RR 1.89 [95% CI 1.67-2.15] skin cancer (table 1).
Discussion: Advanced IBD therapies are commonly used to treat patients with Crohn’s disease and ulcerative colitis. Our data shows that the risk of serious opportunistic infections including herpes zoster infections was highest with JAK inhibitors and thiopurines (RR >2) and thiopurine use is associated with increased risk of non-melanoma skin cancers. Our results emphasize the importance of individualizing therapies for patients with IBD not only based on the disease severity and distribution but also on the adverse risk profile.
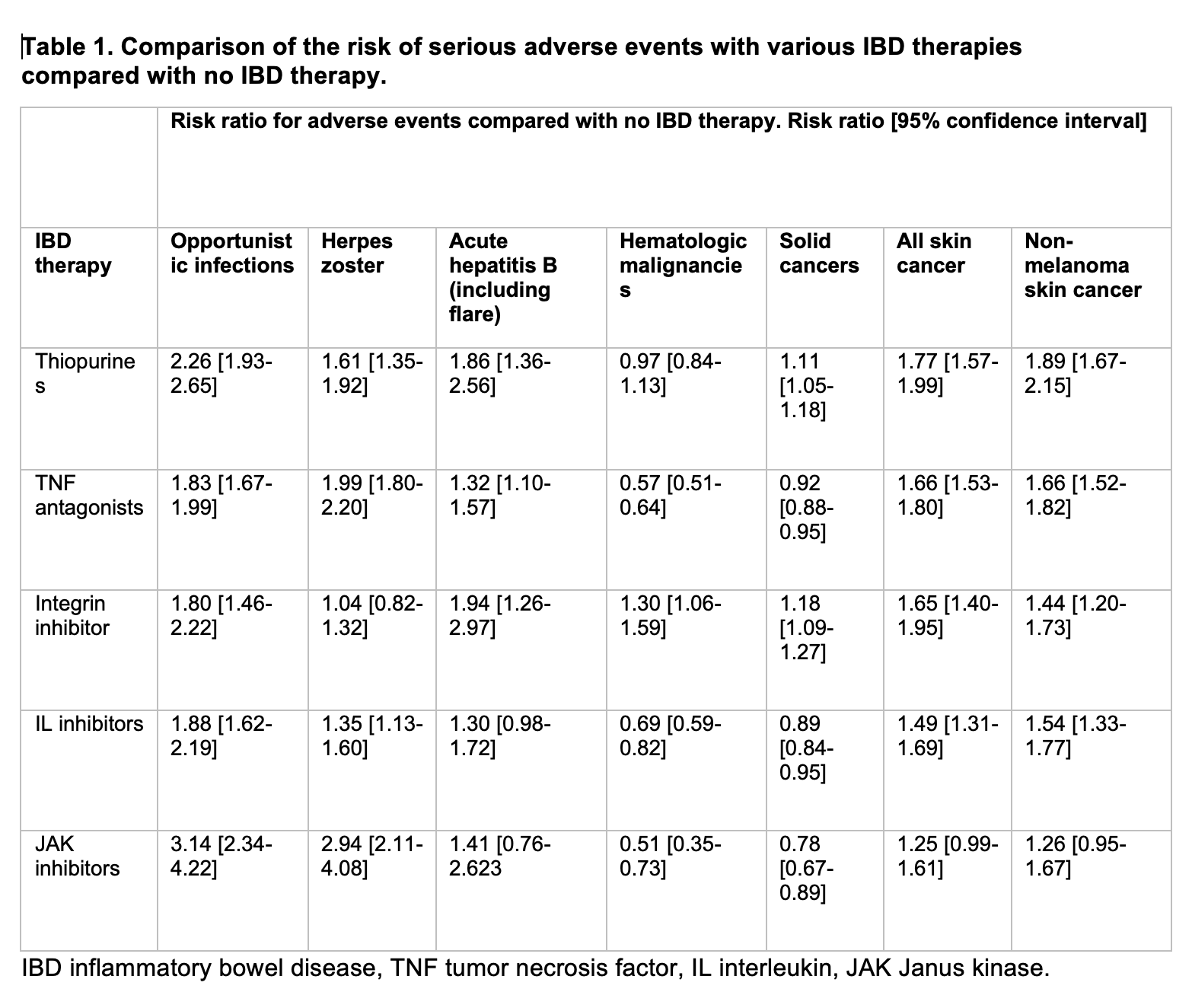
Figure: Table 1. Comparison of the risk of serious adverse events with various IBD therapies compared with no IBD therapy.
Disclosures:
Ritu Singh indicated no relevant financial relationships.
Muhammad Sheharyar Warraich indicated no relevant financial relationships.
Hasan Shoaib indicated no relevant financial relationships.
Nikhil Kalva indicated no relevant financial relationships.
Ritu Singh, MD1, Muhammad Sheharyar Warraich, MD2, Hasan Shoaib, MD1, Nikhil Kalva, MD1. P3208 - Assessment of Serious Adverse Events in Patients Treated With Advanced Inflammatory Bowel Disease Therapies in a Real-World Cohort, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Illinois College of Medicine, Peoria, IL; 2University of Illinois, Peoria, IL
Introduction: There have been phenomenal advancements in the treatment of moderate to severe inflammatory bowel diseases (IBD) over the last decade with improvement in disease remission rates and reduction in complications. However, use of advanced IBD therapies is associated with risk of adverse events ranging from opportunistic infections to solid and hematologic malignancies. There is limited real-world data on the safety of advanced IBD therapies. We aim to explore the real-world data on the serious adverse events associated with the use of advanced therapies.
Methods: We performed a retrospective cohort study utilizing a large research network (TriNetX). Adults with IBD from January 2011 to December 2023 were identified using ICD-10-CM codes for Crohn’s disease and ulcerative colitis. Advanced IBD therapies (TNF inhibitors, integrin inhibitors, interleukin inhibitors, JAK inhibitors) and thiopurines (azathioprine, 6-mercaptoprine) were identified using RxNorm codes. The primary outcome was the rate of opportunistic infections, acute hepatitis B or flare of acute hepatitis B, hematologic malignancies, all skin cancers and non-melanoma skin cancer, and all solid malignancies in patients with IBD treated with thiopurines and individual advanced IBD therapies compared to none of the above therapies.
Results: We identified 570,103 patients with IBD (median follow 1095 days). Among all IBD patients, 89,716 (15.74%) patients were treated with TNF inhibitors, 28,712 (5.04%) with IL-inhibitors, 24,095 with thiopurines (4.23%), 23,980 (4.21%) with integrin inhibitors and 7,627 (1.34%) were treated with JAK inhibitors. The risk of opportunistic infections was highest in patients on JAK inhibitors (RR 3.14 [95% CI 2.34-4.22]) and thiopurines (risk ratio, RR 2.26 [95% confidence interval, CI 1.93-2.65]). Thiopurine use was associated with the highest risk of all skin cancers (RR 1.77 [95% CI 1.57-1.99] and non-melanoma (RR 1.89 [95% CI 1.67-2.15] skin cancer (table 1).
Discussion: Advanced IBD therapies are commonly used to treat patients with Crohn’s disease and ulcerative colitis. Our data shows that the risk of serious opportunistic infections including herpes zoster infections was highest with JAK inhibitors and thiopurines (RR >2) and thiopurine use is associated with increased risk of non-melanoma skin cancers. Our results emphasize the importance of individualizing therapies for patients with IBD not only based on the disease severity and distribution but also on the adverse risk profile.

Figure: Table 1. Comparison of the risk of serious adverse events with various IBD therapies compared with no IBD therapy.
Disclosures:
Ritu Singh indicated no relevant financial relationships.
Muhammad Sheharyar Warraich indicated no relevant financial relationships.
Hasan Shoaib indicated no relevant financial relationships.
Nikhil Kalva indicated no relevant financial relationships.
Ritu Singh, MD1, Muhammad Sheharyar Warraich, MD2, Hasan Shoaib, MD1, Nikhil Kalva, MD1. P3208 - Assessment of Serious Adverse Events in Patients Treated With Advanced Inflammatory Bowel Disease Therapies in a Real-World Cohort, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
