Monday Poster Session
Category: IBD
P3282 - Comprehensive Analysis of Reported Adverse Events for Risankizumab-Rzaa, Ustekinumab, and Adalimumab in Crohn’s Disease: Unveiling Real-World Safety Insights From Pharmacovigilance Database
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Yash P. Ashara, MBBS (he/him/his)
Detroit Medical Center/Wayne State University
Detroit, MI
Presenting Author(s)
Yash P. Ashara, MBBS1, Esha Garg, BS2, Angy Hanna, MD3, Bhavtosh Dedania, MD4
1Detroit Medical Center/Wayne State University, Detroit, MI; 2Wayne State School of Medicine, Detroit, MI; 3John D. Dingell VA Medical Center, Detroit, MI; 4HCA Florida Healthcare, Brandon, FL
Introduction: Crohn’s disease (CD) is a chronic inflammatory disorder of gastrointestinal tract. Advances in biologics have introduced targeted immune therapies. While adalimumab (TNF-α inhibitor) has been mainstay, novel biologics like risankizumab-rzaa (IL-23 inhibitor) and ustekinumab (IL-12/IL-23 blockade) have gained prominence. With increasing options, balancing efficacy and safety is critical. Our study aims to compare safety profiles of risankizumab-rzaa and ustekinumab relative to adalimumab in CD to support evidence-based decision making.
Methods: The US Food and Drug Administration’s Adverse Event Reports System (FAERS) database was queried to identify adverse events of risankizumab-rzaa, ustekinumab and adalimumab in CD. Only cases with known age, sex, and drug exposure were included. Data collected was injury, infection, nervous, musculoskeletal, skin, respiratory disorders. Statistical significance was determined with a p-value of < 0.05, and 95% confidence intervals (CIs).
Results: A total of 99,620 reported adverse event in CD patients treated with risankizumab-rzaa, ustekinumab and adalimumab until September 30, 2024, was. 37.15% were male. Patients on risankizumab-rzaa were 1.32 times more likely to be hospitalized than those on adalimumab [95% CI 1.25-1.39], while no significant difference was observed between ustekinumab and adalimumab. Both biologics had higher serious events than adalimumab. Death risk was 31% lower with ustekinumab compared to adalimumab. Risankizumab-rzaa had lower risk of injury, poisoning, and procedural complications (falls), musculoskeletal (arthralgia, back pain), skin (pruritus, rash), and respiratory (dyspnea, cough) disorders than adalimumab. In contrast, ustekinumab had nearly double the rate of injury, procedural complications (infusion reaction, schedule errors) and a 60% higher infection risk (pneumonia, lower respiratory tract infections) than adalimumab, but it was associated with lower rates of nervous, musculoskeletal, skin and respiratory.
Discussion: CD patients taking risankizumab-rzaa had significantly higher rates of hospitalization but lower rates of injury, musculoskeletal, skin, and respiratory disorders compared to adalimumab. Ustekinumab was associated with lower death risk than adalimumab, but higher rates of injury and infection. These findings offer key insights into the safety profiles of these therapies, aiding in tailoring decisions based on individual patient risk factors.
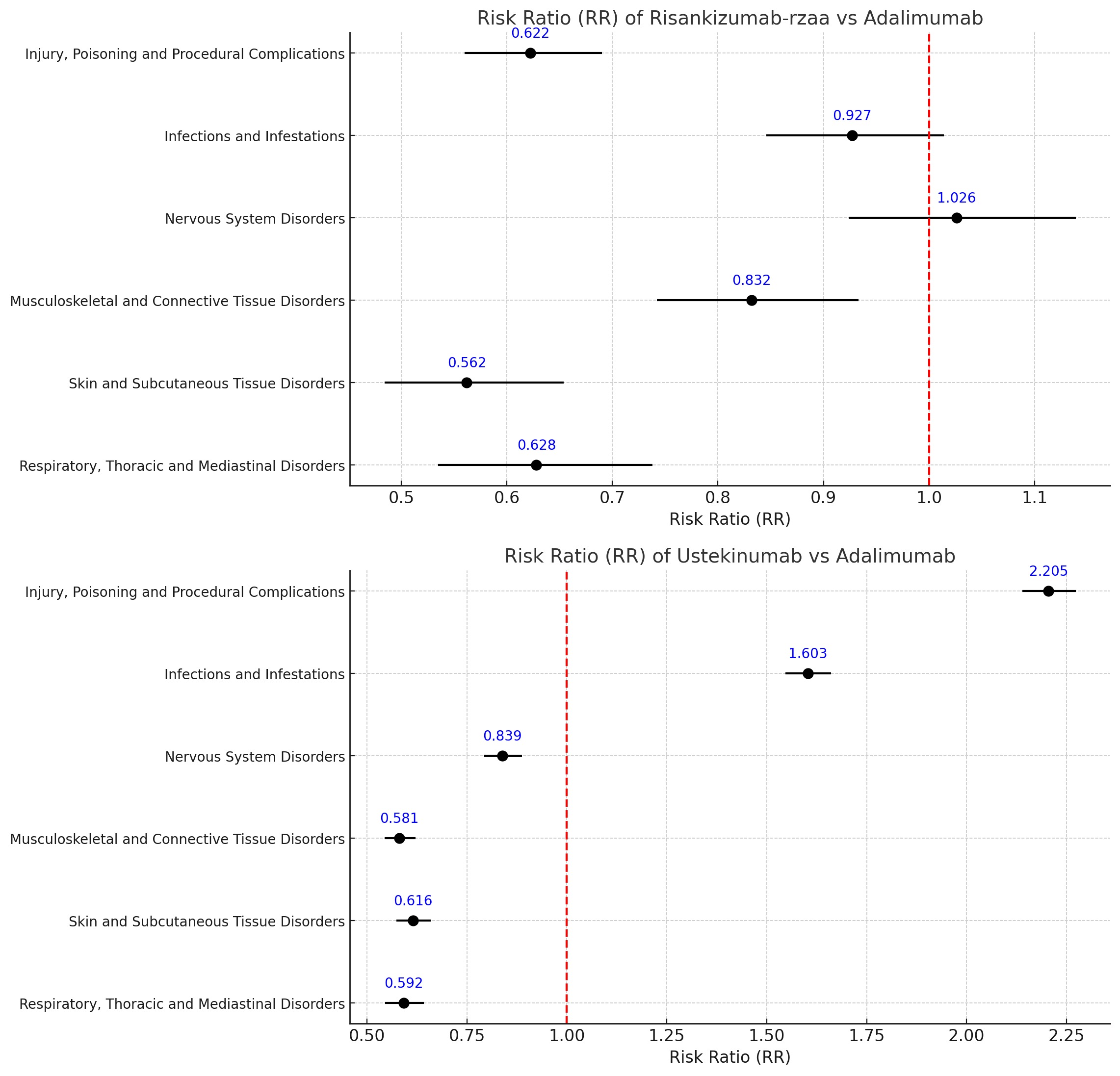
Figure: Forest Plot of Risk Ration of Risankizumab-rzaa vs Adalimumab and Ustekinumab vs Adalimumab

Figure: Bar Graph of Serious Events, Hospitalization, Death in Riskankizumab-rzaa and Ustekinumab with respect to Adalimumab
Disclosures:
Yash Ashara indicated no relevant financial relationships.
Esha Garg indicated no relevant financial relationships.
Angy Hanna indicated no relevant financial relationships.
Bhavtosh Dedania indicated no relevant financial relationships.
Yash P. Ashara, MBBS1, Esha Garg, BS2, Angy Hanna, MD3, Bhavtosh Dedania, MD4. P3282 - Comprehensive Analysis of Reported Adverse Events for Risankizumab-Rzaa, Ustekinumab, and Adalimumab in Crohn’s Disease: Unveiling Real-World Safety Insights From Pharmacovigilance Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Detroit Medical Center/Wayne State University, Detroit, MI; 2Wayne State School of Medicine, Detroit, MI; 3John D. Dingell VA Medical Center, Detroit, MI; 4HCA Florida Healthcare, Brandon, FL
Introduction: Crohn’s disease (CD) is a chronic inflammatory disorder of gastrointestinal tract. Advances in biologics have introduced targeted immune therapies. While adalimumab (TNF-α inhibitor) has been mainstay, novel biologics like risankizumab-rzaa (IL-23 inhibitor) and ustekinumab (IL-12/IL-23 blockade) have gained prominence. With increasing options, balancing efficacy and safety is critical. Our study aims to compare safety profiles of risankizumab-rzaa and ustekinumab relative to adalimumab in CD to support evidence-based decision making.
Methods: The US Food and Drug Administration’s Adverse Event Reports System (FAERS) database was queried to identify adverse events of risankizumab-rzaa, ustekinumab and adalimumab in CD. Only cases with known age, sex, and drug exposure were included. Data collected was injury, infection, nervous, musculoskeletal, skin, respiratory disorders. Statistical significance was determined with a p-value of < 0.05, and 95% confidence intervals (CIs).
Results: A total of 99,620 reported adverse event in CD patients treated with risankizumab-rzaa, ustekinumab and adalimumab until September 30, 2024, was. 37.15% were male. Patients on risankizumab-rzaa were 1.32 times more likely to be hospitalized than those on adalimumab [95% CI 1.25-1.39], while no significant difference was observed between ustekinumab and adalimumab. Both biologics had higher serious events than adalimumab. Death risk was 31% lower with ustekinumab compared to adalimumab. Risankizumab-rzaa had lower risk of injury, poisoning, and procedural complications (falls), musculoskeletal (arthralgia, back pain), skin (pruritus, rash), and respiratory (dyspnea, cough) disorders than adalimumab. In contrast, ustekinumab had nearly double the rate of injury, procedural complications (infusion reaction, schedule errors) and a 60% higher infection risk (pneumonia, lower respiratory tract infections) than adalimumab, but it was associated with lower rates of nervous, musculoskeletal, skin and respiratory.
Discussion: CD patients taking risankizumab-rzaa had significantly higher rates of hospitalization but lower rates of injury, musculoskeletal, skin, and respiratory disorders compared to adalimumab. Ustekinumab was associated with lower death risk than adalimumab, but higher rates of injury and infection. These findings offer key insights into the safety profiles of these therapies, aiding in tailoring decisions based on individual patient risk factors.

Figure: Forest Plot of Risk Ration of Risankizumab-rzaa vs Adalimumab and Ustekinumab vs Adalimumab

Figure: Bar Graph of Serious Events, Hospitalization, Death in Riskankizumab-rzaa and Ustekinumab with respect to Adalimumab
Disclosures:
Yash Ashara indicated no relevant financial relationships.
Esha Garg indicated no relevant financial relationships.
Angy Hanna indicated no relevant financial relationships.
Bhavtosh Dedania indicated no relevant financial relationships.
Yash P. Ashara, MBBS1, Esha Garg, BS2, Angy Hanna, MD3, Bhavtosh Dedania, MD4. P3282 - Comprehensive Analysis of Reported Adverse Events for Risankizumab-Rzaa, Ustekinumab, and Adalimumab in Crohn’s Disease: Unveiling Real-World Safety Insights From Pharmacovigilance Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
