Monday Poster Session
Category: Infections and Microbiome
P3478 - Persistent Gastrointestinal Dysbiosis in a Male Patient With Post-Acute COVID-19 Syndrome: A Case Report Highlighting Microbiome-Related Sequelae
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Jennifer John, MS (she/her/hers)
A.T. Still University
Little Falls, NJ
Presenting Author(s)
Jennifer John, MS1, Nancy Lentine, DO2
1A.T. Still University, Little Falls, NJ; 2Dr. Nancy Lentine Integrative Family Medicine, Little Falls, NJ
Introduction: Post-acute COVID-19 syndrome (PACS), or long COVID, refers to symptoms lasting beyond four weeks after infection. Fatigue, cognitive impairment, and gastrointestinal (GI) symptoms such as bloating, food sensitivities, and altered bowel habits are common, often despite normal diagnostic testing. Disruption of the gut microbiome is increasingly recognized as a potential contributor through immune dysregulation, inflammation, and gut–brain signaling.
Case Description/
Methods: A 50-year-old man with no prior GI or autoimmune history presented with fatigue, brain fog, bloating, and new food intolerances after eight separate SARS-CoV-2 infections. Despite recovery from the acute illness, he experienced persistent GI symptoms and cognitive changes. Standard labs and imaging were unremarkable. Functional stool testing showed Helicobacter pylori, bacterial overgrowth, low pancreatic elastase, decreased beneficial bacteria, elevated anti-gliadin and secretory IgA, and a low Firmicutes:Bacteroidetes ratio. He was treated with nystatin, probiotics, enzymes, and a gluten-free, low-sugar diet. Within eight weeks, he reported improved energy, mental clarity, GI function, and food tolerance without adverse effects.
Discussion: This case illustrates the potential role of intestinal dysbiosis in the pathophysiology of PACS. The patient’s altered gut microbiota—marked by a low Firmicutes:Bacteroidetes ratio and loss of short-chain fatty acid–producing bacteria—likely contributed to systemic inflammation and gut barrier dysfunction. Elevated anti-gliadin and secretory IgA, along with new food intolerances, suggest an acquired immune response driven by microbiota shifts and increased permeability. The presence of antibiotic resistance genes indicates long-term microbial disruption, with implications for future treatment and antibiotic stewardship. This supports the value of functional stool testing in PACS evaluation, especially for patients with persistent GI symptoms. The patient’s improvement with targeted microbiome therapy highlights the potential of gut-directed care in long COVID. Although this is a single case without follow-up testing, the timing of symptom resolution after intervention supports further study.
This case underscores how personalized, microbiome-informed therapy may benefit PACS patients and highlights the role of gastroenterologists in long COVID care. When standard testing is inconclusive, advanced microbiome analysis may help identify modifiable contributors to ongoing symptoms.
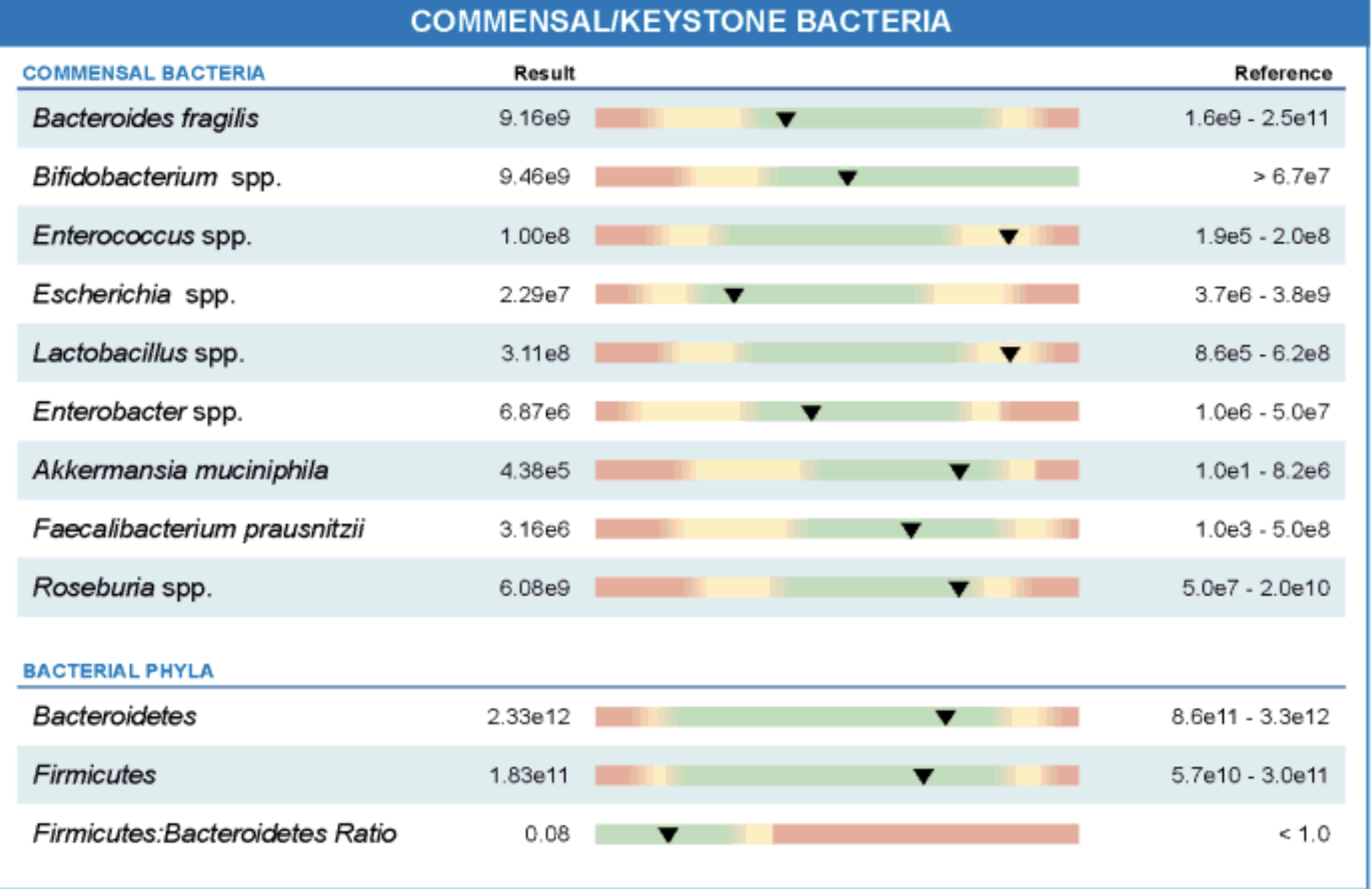
Figure: Stool analysis revealed a significantly reduced Firmicutes:Bacteroidetes ratio (0.08), indicating microbial imbalance. Notably, Faecalibacterium prausnitzii, Roseburia spp., and Akkermansia muciniphila were borderline low or below optimal ranges, suggesting reduced levels of beneficial, anti-inflammatory bacteria. Although total Bacteroides fragilis and Bifidobacterium spp. were within normal range, the presence of elevated Enterococcus spp. and Enterobacter spp. pointed to opportunistic bacterial overgrowth.
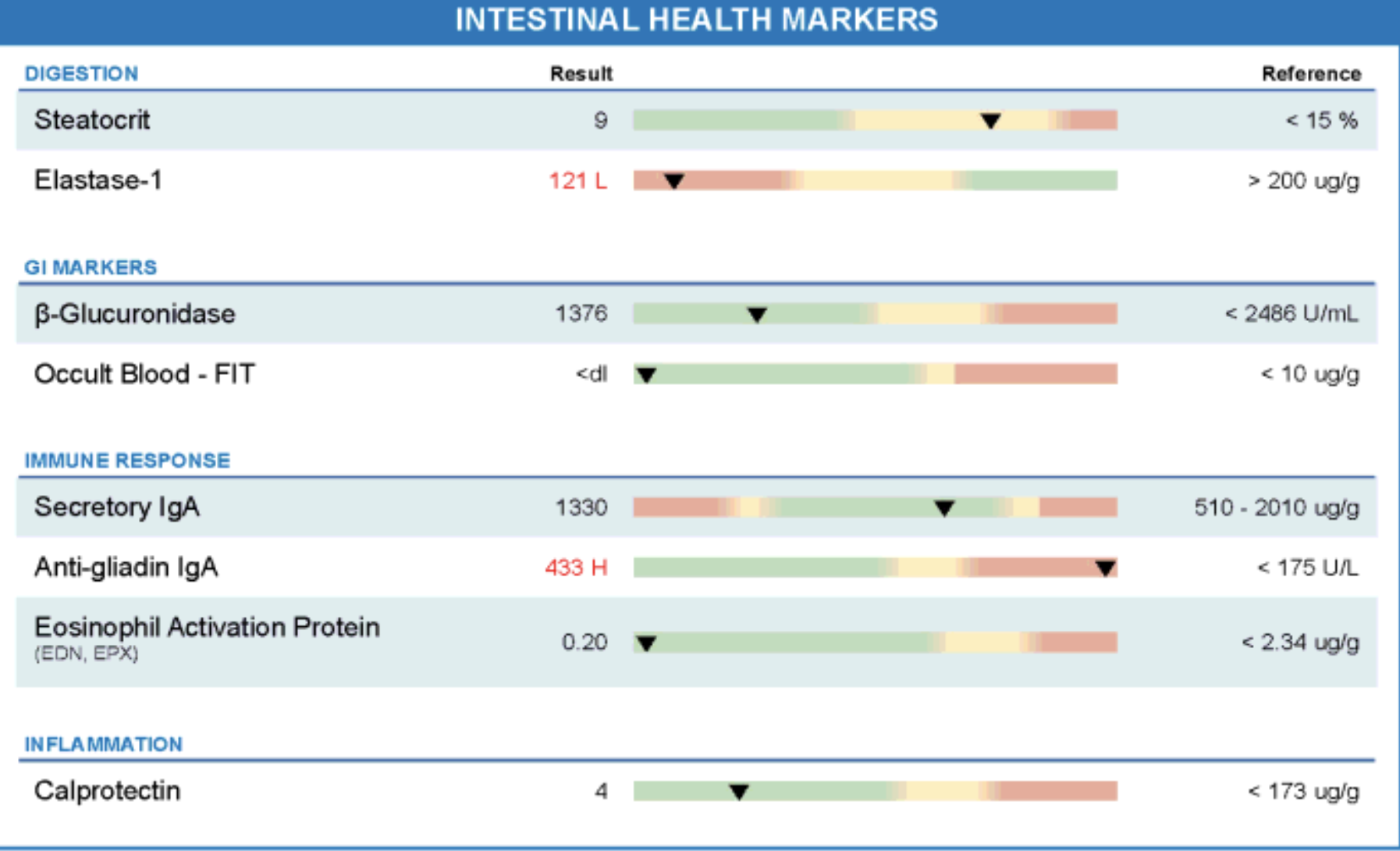
Figure: Functional stool testing revealed pancreatic elastase-1 was markedly low (121 µg/g; reference >200), indicating exocrine pancreatic insufficiency. Immune markers showed elevated secretory IgA (1330) and significantly increased anti-gliadin IgA (433; reference <175), suggesting mucosal immune activation and gluten sensitivity. Calprotectin and eosinophil activation protein were within normal range, ruling out active intestinal inflammation. Occult blood was undetectable, and β-glucuronidase was in the expected range.
Disclosures:
Jennifer John indicated no relevant financial relationships.
Nancy Lentine indicated no relevant financial relationships.
Jennifer John, MS1, Nancy Lentine, DO2. P3478 - Persistent Gastrointestinal Dysbiosis in a Male Patient With Post-Acute COVID-19 Syndrome: A Case Report Highlighting Microbiome-Related Sequelae, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1A.T. Still University, Little Falls, NJ; 2Dr. Nancy Lentine Integrative Family Medicine, Little Falls, NJ
Introduction: Post-acute COVID-19 syndrome (PACS), or long COVID, refers to symptoms lasting beyond four weeks after infection. Fatigue, cognitive impairment, and gastrointestinal (GI) symptoms such as bloating, food sensitivities, and altered bowel habits are common, often despite normal diagnostic testing. Disruption of the gut microbiome is increasingly recognized as a potential contributor through immune dysregulation, inflammation, and gut–brain signaling.
Case Description/
Methods: A 50-year-old man with no prior GI or autoimmune history presented with fatigue, brain fog, bloating, and new food intolerances after eight separate SARS-CoV-2 infections. Despite recovery from the acute illness, he experienced persistent GI symptoms and cognitive changes. Standard labs and imaging were unremarkable. Functional stool testing showed Helicobacter pylori, bacterial overgrowth, low pancreatic elastase, decreased beneficial bacteria, elevated anti-gliadin and secretory IgA, and a low Firmicutes:Bacteroidetes ratio. He was treated with nystatin, probiotics, enzymes, and a gluten-free, low-sugar diet. Within eight weeks, he reported improved energy, mental clarity, GI function, and food tolerance without adverse effects.
Discussion: This case illustrates the potential role of intestinal dysbiosis in the pathophysiology of PACS. The patient’s altered gut microbiota—marked by a low Firmicutes:Bacteroidetes ratio and loss of short-chain fatty acid–producing bacteria—likely contributed to systemic inflammation and gut barrier dysfunction. Elevated anti-gliadin and secretory IgA, along with new food intolerances, suggest an acquired immune response driven by microbiota shifts and increased permeability. The presence of antibiotic resistance genes indicates long-term microbial disruption, with implications for future treatment and antibiotic stewardship. This supports the value of functional stool testing in PACS evaluation, especially for patients with persistent GI symptoms. The patient’s improvement with targeted microbiome therapy highlights the potential of gut-directed care in long COVID. Although this is a single case without follow-up testing, the timing of symptom resolution after intervention supports further study.
This case underscores how personalized, microbiome-informed therapy may benefit PACS patients and highlights the role of gastroenterologists in long COVID care. When standard testing is inconclusive, advanced microbiome analysis may help identify modifiable contributors to ongoing symptoms.

Figure: Stool analysis revealed a significantly reduced Firmicutes:Bacteroidetes ratio (0.08), indicating microbial imbalance. Notably, Faecalibacterium prausnitzii, Roseburia spp., and Akkermansia muciniphila were borderline low or below optimal ranges, suggesting reduced levels of beneficial, anti-inflammatory bacteria. Although total Bacteroides fragilis and Bifidobacterium spp. were within normal range, the presence of elevated Enterococcus spp. and Enterobacter spp. pointed to opportunistic bacterial overgrowth.

Figure: Functional stool testing revealed pancreatic elastase-1 was markedly low (121 µg/g; reference >200), indicating exocrine pancreatic insufficiency. Immune markers showed elevated secretory IgA (1330) and significantly increased anti-gliadin IgA (433; reference <175), suggesting mucosal immune activation and gluten sensitivity. Calprotectin and eosinophil activation protein were within normal range, ruling out active intestinal inflammation. Occult blood was undetectable, and β-glucuronidase was in the expected range.
Disclosures:
Jennifer John indicated no relevant financial relationships.
Nancy Lentine indicated no relevant financial relationships.
Jennifer John, MS1, Nancy Lentine, DO2. P3478 - Persistent Gastrointestinal Dysbiosis in a Male Patient With Post-Acute COVID-19 Syndrome: A Case Report Highlighting Microbiome-Related Sequelae, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
