Monday Poster Session
Category: Interventional Endoscopy
P3547 - Efficacy and Safety of Anti-Reflux Mucosal Ablation in Proton Pump Inhibitors Refractory Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Ammad Javaid Chaudhary, MD
Henry Ford Health
Detroit, MI
Presenting Author(s)
Ammad Javaid. Chaudhary, MD1, Zain Ul Abideen, MBBS2, Muhammad Hassan Waseem, MBBS3, Sania Aimen, MBBS4, Noor Ul Huda Ramzan, MD5, Hafsa Shahid, MBBS6, Muhammad Shahzil, MD7, Usman Bin Hameed, MD8, Samreen Zafar, MD9, Cyrus Piraka, MD1, Brian Ginnebaugh, MD1
1Henry Ford Health, Detroit, MI; 2King Edward Medical University, Lahore, Punjab, Pakistan; 3Allama Iqbal Medical College, Lahore, Punjab, Pakistan; 4Quetta Institute of Medical Sciences, Quetta, Balochistan, Pakistan; 5University of Texas Southwestern Medical Center, Dallas, TX; 6Brigham and Women's Hospital, Detroit, MI; 7Penn State Health Milton S. Hershey Medical Center, Hershey, PA; 8Corewell Health William Beaumont University Hospital, Royal Oak, MI; 9Lake Huron Medical Center, Port Huron, MI
Introduction: Gastroesophageal reflux disease (GERD) refractory to the Proton pump inhibitors (PPI) poses a serious clinical challenge. New endoscopic minimally invasive procedures, causing cardiac remodeling, have emerged. This meta-analysis aimed to evaluate the efficacy and safety of the less invasive approach called Anti-reflux mucosal ablation (ARMA) in GERD.
Methods: Electronic databases including PubMed, Cochrane Central and ScienceDirect were searched from inception till January 2025. This review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The pooled analysis was performed using a random effects model in R version 4.2.3 employing the “metaprop” package. The primary and secondary outcomes of interest were clinical success rate at short-term (< 1 year) and long-term (≥ 1 year) follow-ups, adverse events, dysphagia requiring dilation and Gastroesophageal reflux disease health-related quality of life (GERD-HRQL) score. The quality assessment was done by the Newcastle Ottawa Scale. Publication bias was assessed visually through funnel plots.
Results: Five studies encompassing a total of 294 patients were included in this meta-analysis. The pooled clinical success rates at short and long follow-ups were 72 % (95% CI:[56-84%]; I2=70.8%) and 73 % (95% CI:[19-97%]; I2=0%) respectively. The pooled adverse effects were 18% (95%CI:[4-54%]; I2=91.6%). Among these adverse effects, the dysphagia requiring dilation was 13% (95%CI:[7-22%]; I2=0%).
Discussion: This meta-analysis showed that ARMA has a high clinical success rate along with low incidence of adverse effects implying that it is a safe and effective minimally invasive endoscopic treatment modality for GERD resistant to PPI.
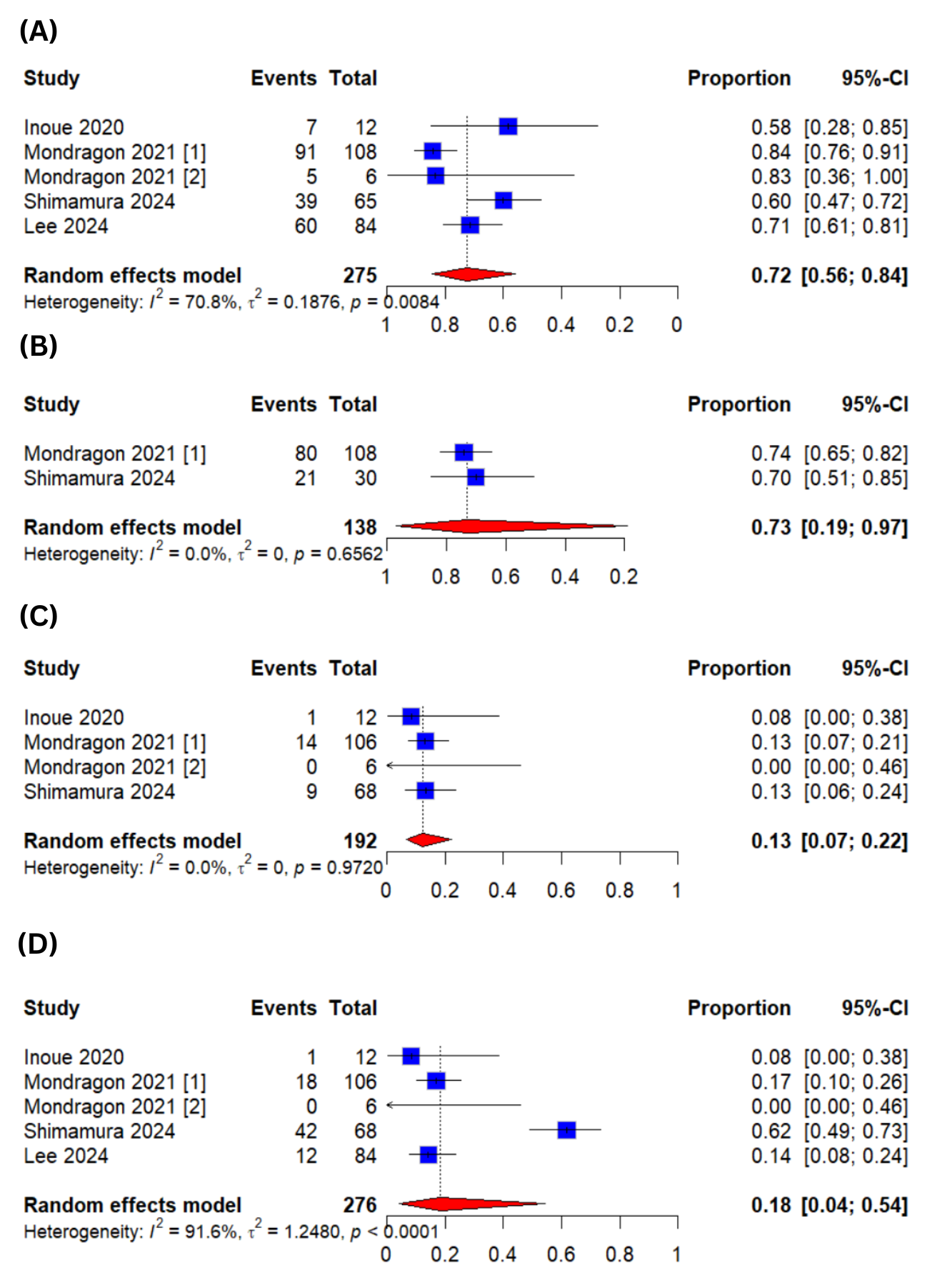
Figure: Figure 1: Forest Plots for (A)Clinical Success at Short Term Follow-up (B)Clinical Success at Long Term Follow-up (C)Dysphagia Requiring Dilation (D)Adverse Events
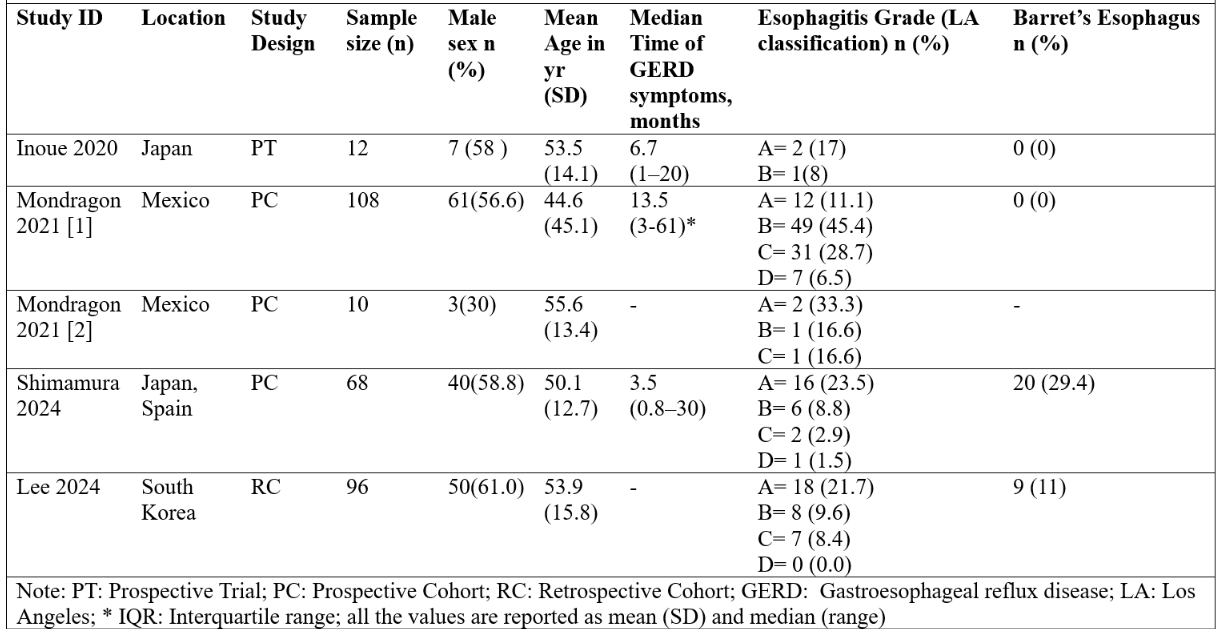
Figure: Figure 2: Baseline Characteristics of the Included Studies
Disclosures:
Ammad Chaudhary indicated no relevant financial relationships.
Zain Ul Abideen indicated no relevant financial relationships.
Muhammad Hassan Waseem indicated no relevant financial relationships.
Sania Aimen indicated no relevant financial relationships.
Noor Ul Huda Ramzan indicated no relevant financial relationships.
Hafsa Shahid indicated no relevant financial relationships.
Muhammad Shahzil indicated no relevant financial relationships.
Usman Bin Hameed indicated no relevant financial relationships.
Samreen Zafar indicated no relevant financial relationships.
Cyrus Piraka indicated no relevant financial relationships.
Brian Ginnebaugh: Ardelyx – Speakers Bureau.
Ammad Javaid. Chaudhary, MD1, Zain Ul Abideen, MBBS2, Muhammad Hassan Waseem, MBBS3, Sania Aimen, MBBS4, Noor Ul Huda Ramzan, MD5, Hafsa Shahid, MBBS6, Muhammad Shahzil, MD7, Usman Bin Hameed, MD8, Samreen Zafar, MD9, Cyrus Piraka, MD1, Brian Ginnebaugh, MD1. P3547 - Efficacy and Safety of Anti-Reflux Mucosal Ablation in Proton Pump Inhibitors Refractory Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Henry Ford Health, Detroit, MI; 2King Edward Medical University, Lahore, Punjab, Pakistan; 3Allama Iqbal Medical College, Lahore, Punjab, Pakistan; 4Quetta Institute of Medical Sciences, Quetta, Balochistan, Pakistan; 5University of Texas Southwestern Medical Center, Dallas, TX; 6Brigham and Women's Hospital, Detroit, MI; 7Penn State Health Milton S. Hershey Medical Center, Hershey, PA; 8Corewell Health William Beaumont University Hospital, Royal Oak, MI; 9Lake Huron Medical Center, Port Huron, MI
Introduction: Gastroesophageal reflux disease (GERD) refractory to the Proton pump inhibitors (PPI) poses a serious clinical challenge. New endoscopic minimally invasive procedures, causing cardiac remodeling, have emerged. This meta-analysis aimed to evaluate the efficacy and safety of the less invasive approach called Anti-reflux mucosal ablation (ARMA) in GERD.
Methods: Electronic databases including PubMed, Cochrane Central and ScienceDirect were searched from inception till January 2025. This review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The pooled analysis was performed using a random effects model in R version 4.2.3 employing the “metaprop” package. The primary and secondary outcomes of interest were clinical success rate at short-term (< 1 year) and long-term (≥ 1 year) follow-ups, adverse events, dysphagia requiring dilation and Gastroesophageal reflux disease health-related quality of life (GERD-HRQL) score. The quality assessment was done by the Newcastle Ottawa Scale. Publication bias was assessed visually through funnel plots.
Results: Five studies encompassing a total of 294 patients were included in this meta-analysis. The pooled clinical success rates at short and long follow-ups were 72 % (95% CI:[56-84%]; I2=70.8%) and 73 % (95% CI:[19-97%]; I2=0%) respectively. The pooled adverse effects were 18% (95%CI:[4-54%]; I2=91.6%). Among these adverse effects, the dysphagia requiring dilation was 13% (95%CI:[7-22%]; I2=0%).
Discussion: This meta-analysis showed that ARMA has a high clinical success rate along with low incidence of adverse effects implying that it is a safe and effective minimally invasive endoscopic treatment modality for GERD resistant to PPI.

Figure: Figure 1: Forest Plots for (A)Clinical Success at Short Term Follow-up (B)Clinical Success at Long Term Follow-up (C)Dysphagia Requiring Dilation (D)Adverse Events

Figure: Figure 2: Baseline Characteristics of the Included Studies
Disclosures:
Ammad Chaudhary indicated no relevant financial relationships.
Zain Ul Abideen indicated no relevant financial relationships.
Muhammad Hassan Waseem indicated no relevant financial relationships.
Sania Aimen indicated no relevant financial relationships.
Noor Ul Huda Ramzan indicated no relevant financial relationships.
Hafsa Shahid indicated no relevant financial relationships.
Muhammad Shahzil indicated no relevant financial relationships.
Usman Bin Hameed indicated no relevant financial relationships.
Samreen Zafar indicated no relevant financial relationships.
Cyrus Piraka indicated no relevant financial relationships.
Brian Ginnebaugh: Ardelyx – Speakers Bureau.
Ammad Javaid. Chaudhary, MD1, Zain Ul Abideen, MBBS2, Muhammad Hassan Waseem, MBBS3, Sania Aimen, MBBS4, Noor Ul Huda Ramzan, MD5, Hafsa Shahid, MBBS6, Muhammad Shahzil, MD7, Usman Bin Hameed, MD8, Samreen Zafar, MD9, Cyrus Piraka, MD1, Brian Ginnebaugh, MD1. P3547 - Efficacy and Safety of Anti-Reflux Mucosal Ablation in Proton Pump Inhibitors Refractory Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
