Monday Poster Session
Category: Liver
P3720 - GLP-1 Receptor Agonist Use Is Associated With Lower Risks of Cardiovascular and Kidney Outcomes in Type 2 Diabetic Liver Transplant Recipients
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Shu-Yen Emily Chan, MD (she/her/hers)
Weiss Memorial Hospital
Nashville, TN
Presenting Author(s)
Emily Shu-Yen Chan, MD1, Natchaya Polpichai, MD2, Pojsakorn Danpanichkul, MD3, Rebecca Jen-Ling Hsieh, MD4, Patrick Twohig, MD5
1Weiss Memorial Hospital, Chicago, IL; 2Division of gastroenterology/hepatology, Medical College of Georgia at Augusta University, Chicago, IL; 3Department of Internal Medicine, Texas Tech University Healt, Lubbock, TX; 4Danbury Hospital, Danbury, CT; 5University of Rochester Medical Center, Rochester, NY
Introduction: Cardiovascular disease remains a leading cause of post‐transplant mortality among liver transplant (LT) recipients after acute phase, particularly in those with diabetes. Glucagon‐like peptide‐1 receptor agonists (GLP‑1 RAs) have demonstrated both cardiovascular and renal benefits in the general population with type 2 diabetes mellitus (T2DM), their effects in diabetic LT recipients remain unclear.
Methods: We performed a retrospective cohort study using US collaborative Network within TriNetX, from 1/1/2014 to 12/31/ 2023. Adult LT recipients (≥ 18 years) with preexisting type 2 diabetes who started a GLP‑1 RA within six months post‐transplant were compared to propensity‑score matched diabetic recipients who did not receive GLP‑1 RAs. Recipients who died within three months or had incomplete follow‑up were excluded. Matching factors included demographics, comorbidities, lab values, and concurrent medications. The primary outcome was all‑cause mortality within five years. Secondary outcomes were new major adverse cardiovascular events (MACEs) and major adverse kidney events (MAKEs), defined as new dialysis dependence. Subgroup analyses were stratified by BMI (< 30 vs. ≥ 30). Adjusted hazard ratios and 95% confidence intervals were estimated using Cox models, with proportional hazards assumptions tested by the Schoenfeld method. Negative control outcomes were herniated discs and trauma.
Results: Among 31,391 LT recipients with T2DM, 2,507 (8%) initiated a GLP‑1 RA within six months post‑transplant. After PSM, 2,274 GLP‑1 RA users (mean [SD] age, 60.3 ± 10.2 years; 37.7% female) were compared 1:1 with non‑users (60.1 ± 10.8 years; 37.1% female). Over a median follow‑up of three years, GLP-1 RAs users were associated with significant lower risks of mortality (aHR:0.448; 95% CI 0.377–0.534), a composite of MACEs (aHR:0.719; 95% CI 0.594–0.869) including acute myocardial infarction (aHR:0.748; 95% CI, 0.578-0.967), heart failure (aHR :0.802; 95% CI, 0.654-0.984), and MAKEs (aHR:0.503; 95% CI 0.381–0.664) compared with GLP-1 RAs non-users. No significant differences were found in stroke including cerebral infarction or nontraumatic brain injury (aHR:0.757; 95% CI, 0.556–1.031) nor negative outcome controls including trauma (aHR: 0.865; 95% CI, 0.653-1.144) and HIVD (aHR: 1.177; 95% CI, 0.984-1.408).
Discussion: In LT patients with T2DM, GLP-1 RAs use was associated with significant reductions in all-cause mortality, MAKEs, and MACEs compared to nonuser.
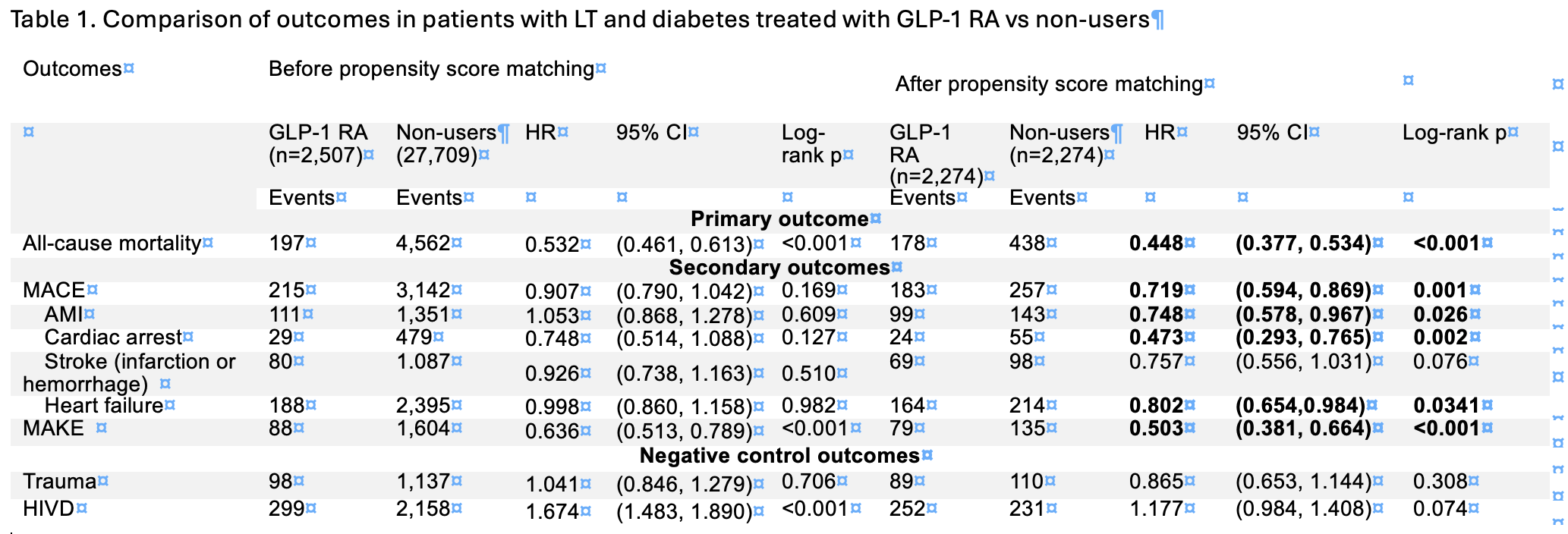
Figure: Table 1. Comparison of outcomes in patients with LT and diabetes treated with GLP-1 RA vs non-users
Disclosures:
Emily Shu-Yen Chan indicated no relevant financial relationships.
Natchaya Polpichai indicated no relevant financial relationships.
Pojsakorn Danpanichkul indicated no relevant financial relationships.
Rebecca Jen-Ling Hsieh indicated no relevant financial relationships.
Patrick Twohig indicated no relevant financial relationships.
Emily Shu-Yen Chan, MD1, Natchaya Polpichai, MD2, Pojsakorn Danpanichkul, MD3, Rebecca Jen-Ling Hsieh, MD4, Patrick Twohig, MD5. P3720 - GLP-1 Receptor Agonist Use Is Associated With Lower Risks of Cardiovascular and Kidney Outcomes in Type 2 Diabetic Liver Transplant Recipients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Weiss Memorial Hospital, Chicago, IL; 2Division of gastroenterology/hepatology, Medical College of Georgia at Augusta University, Chicago, IL; 3Department of Internal Medicine, Texas Tech University Healt, Lubbock, TX; 4Danbury Hospital, Danbury, CT; 5University of Rochester Medical Center, Rochester, NY
Introduction: Cardiovascular disease remains a leading cause of post‐transplant mortality among liver transplant (LT) recipients after acute phase, particularly in those with diabetes. Glucagon‐like peptide‐1 receptor agonists (GLP‑1 RAs) have demonstrated both cardiovascular and renal benefits in the general population with type 2 diabetes mellitus (T2DM), their effects in diabetic LT recipients remain unclear.
Methods: We performed a retrospective cohort study using US collaborative Network within TriNetX, from 1/1/2014 to 12/31/ 2023. Adult LT recipients (≥ 18 years) with preexisting type 2 diabetes who started a GLP‑1 RA within six months post‐transplant were compared to propensity‑score matched diabetic recipients who did not receive GLP‑1 RAs. Recipients who died within three months or had incomplete follow‑up were excluded. Matching factors included demographics, comorbidities, lab values, and concurrent medications. The primary outcome was all‑cause mortality within five years. Secondary outcomes were new major adverse cardiovascular events (MACEs) and major adverse kidney events (MAKEs), defined as new dialysis dependence. Subgroup analyses were stratified by BMI (< 30 vs. ≥ 30). Adjusted hazard ratios and 95% confidence intervals were estimated using Cox models, with proportional hazards assumptions tested by the Schoenfeld method. Negative control outcomes were herniated discs and trauma.
Results: Among 31,391 LT recipients with T2DM, 2,507 (8%) initiated a GLP‑1 RA within six months post‑transplant. After PSM, 2,274 GLP‑1 RA users (mean [SD] age, 60.3 ± 10.2 years; 37.7% female) were compared 1:1 with non‑users (60.1 ± 10.8 years; 37.1% female). Over a median follow‑up of three years, GLP-1 RAs users were associated with significant lower risks of mortality (aHR:0.448; 95% CI 0.377–0.534), a composite of MACEs (aHR:0.719; 95% CI 0.594–0.869) including acute myocardial infarction (aHR:0.748; 95% CI, 0.578-0.967), heart failure (aHR :0.802; 95% CI, 0.654-0.984), and MAKEs (aHR:0.503; 95% CI 0.381–0.664) compared with GLP-1 RAs non-users. No significant differences were found in stroke including cerebral infarction or nontraumatic brain injury (aHR:0.757; 95% CI, 0.556–1.031) nor negative outcome controls including trauma (aHR: 0.865; 95% CI, 0.653-1.144) and HIVD (aHR: 1.177; 95% CI, 0.984-1.408).
Discussion: In LT patients with T2DM, GLP-1 RAs use was associated with significant reductions in all-cause mortality, MAKEs, and MACEs compared to nonuser.

Figure: Table 1. Comparison of outcomes in patients with LT and diabetes treated with GLP-1 RA vs non-users
Disclosures:
Emily Shu-Yen Chan indicated no relevant financial relationships.
Natchaya Polpichai indicated no relevant financial relationships.
Pojsakorn Danpanichkul indicated no relevant financial relationships.
Rebecca Jen-Ling Hsieh indicated no relevant financial relationships.
Patrick Twohig indicated no relevant financial relationships.
Emily Shu-Yen Chan, MD1, Natchaya Polpichai, MD2, Pojsakorn Danpanichkul, MD3, Rebecca Jen-Ling Hsieh, MD4, Patrick Twohig, MD5. P3720 - GLP-1 Receptor Agonist Use Is Associated With Lower Risks of Cardiovascular and Kidney Outcomes in Type 2 Diabetic Liver Transplant Recipients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
