Monday Poster Session
Category: Liver
P3780 - Achieving Undetectable Hepatitis Delta Virus RNA at End of Therapy With Bulevirtide 10 Mg/day With or Without PegIFNα Is Strongly Associated With Posttreatment Virologic Response in Chronic Hepatitis Delta
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Renee-Claude Mercier, PharmD
Gilead Sciences, Inc.
Foster City, CA
Presenting Author(s)
Fabien Zoulim, MD, PhD1, Tarik Asselah, MD, PhD2, Soo Aleman, MD, PhD3, Maurizia R. Brunetto, MD4, Vladimir Chulanov, MD, PhD, DSc5, Adrian Streinu-Cercel, MD, PhD6, George Sebastian Gherlan, MD7, Pavel Bogomolov, MD, PhD8, Tatiana Stepanova, MD9, Viacheslav Morozov, MD, PhD10, Olga Sagalova, PhD11, Renee-Claude Mercier, PharmD12, Lei Ye, PhD12, Amos Lichtman, MD, MPH12, Dmitry Manuilov, MD12, Heiner Wedemeyer, MD13, Pietro Lampertico, MD, PhD14
1Hospital Croix Rousse, Lyon Hepatology Institute, Lyon, Provence-Alpes-Cote d'Azur, France; 2Hôpital Beaujon APHP, Université de Paris, INSERM, Clichy, Ile-de-France, France; 3Department of Infectious Diseases, Karolinska University Hospital/Karolinska Institutet, Stockholm, Stockholms Lan, Sweden; 4Hepatology Unit, Reference Center of the Tuscany Region for Chronic Liver Disease and Cancer, University Hospital of Pisa; Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Toscana, Italy; 5Sechenov University, Moscow, Moskva, Russia; 6National Institute of Infectious Diseases Prof. Dr. Matei Bals; University of Medicine and Pharmacy “Carol Davila” Bucharest, Bucharest, Bucuresti, Romania; 7University of Medicine and Pharmacy “Carol Davila” Bucharest; Dr. Victor Babes Foundation, Bucharest, Bucuresti, Romania; 8M.F. Vladimirsky Moscow Regional Research and Clinical Institute, Moscow, Moskva, Russia; 9LLC Clinic of Modern Medicine, Moscow, Moskva, Russia; 10LLC Medical Company “Hepatolog”, Samara, Samara, Russia; 11South Ural State Medical University, Chelyabinsk, Orel, Russia; 12Gilead Sciences, Inc., Foster City, CA; 13Clinic for Gastroenterology, Hepatology, Infectious Diseases, and Endocrinology, Hannover Medical School, Hannover, Sachsen, Germany; 14Division of Gastroenterology and Hepatology, Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico; CRC “A. M. and A. Migliavacca” Center for Liver Disease, Department of Pathophysiology and Transplantation, University of Milan, Milan, Lombardia, Italy
Introduction: Bulevirtide (BLV) 2 mg is approved for chronic hepatitis delta (CHD) treatment in Europe, Australia, and Russia. In an integrated analysis of BLV 10 mg monotherapy or in combination with pegylated interferon alpha (PegIFNα), we explored whether hepatitis delta virus (HDV) RNA levels undetectable (target not detected; TND) vs below the lower limit of quantitation (< LLOQ; target detected [TD]) at end of treatment (EOT) affect posttreatment (PT) virologic response.
Methods: A pooled analysis of data was performed from patients (pts) who completed 2 or 3 years of BLV 10 mg/day (d) with or without PegIFNα in the MYR204 Phase 2 and MYR301 Phase 3 studies. Pts received (A) BLV 10 mg + PegIFNα for 48 weeks (W) followed by 48W of monotherapy with BLV 10 mg (n = 50), (B) BLV 10 mg for 96W (n = 100), or (C) BLV 10 mg for 144W (n = 50). Pts were followed for up to 48W after EOT (follow-up [FU]48). HDV RNA levels were determined by RT-qPCR using RoboGene 2.0 (LLOQ 50 IU/mL, limit of detection 6 IU/mL). Virologic response rates at FU48 were compared between pts with undetectable HDV RNA and those with HDV RNA < LLOQ, TD at EOT.
Results: Demographics were similar across groups (Table). At EOT, 49% (97/200) achieved undetectable HDV RNA: (A) 70% (35/50), (B) 37% (37/100), and (C) 50% (25/50); 24% (48/200) had < LLOQ, TD: (A) 16% (8/50), (B) 30% (30/100), and (C) 20% (10/50; Figure). At FU48, undetectable HDV RNA was observed in 25% (49/200) overall: (A) 23/50 (46%) of pts receiving combination therapy, (B) 14% (14/100) of pts receiving 2 years of BLV monotherapy, and (C) 24% (12/50) of pts receiving 3 years of BLV monotherapy. Of the pts with undetectable HDV RNA at EOT, 46% (45/97) maintained undetectable HDV RNA at FU48: (A) 60% (21/35), (B) 35% (13/37), and (C) 44% (11/25); 6% (6/97) had < LLOQ, TD at FU48: (A) 6% (2/35), (B) 9% (3/37), and (C) 4% (1/25). Of those who had < LLOQ, TD at EOT, only 6% (3/48) had undetectable HDV RNA (1 per group) and 4% (2/48) remained < LLOQ, TD at FU48, while 71% (34/48) had HDV RNA >LLOQ: (A) 62.5% (5/8), (B) 80% (24/30), and (C) 50% (5/10); during FU, 9 discontinued the study early.
Discussion: In pts with compensated CHD, achieving undetectable HDV RNA with TND at EOT is associated with virologic suppression at 48W PT. Combination therapy with BLV 10 mg/d and PegIFNα and longer treatment with BLV monotherapy were associated with higher rates of undetectable HDV RNA at EOT and FU48. Pts with HDV RNA < LLOQ, TD at EOT are likely to have HDV RNA rebound off therapy.
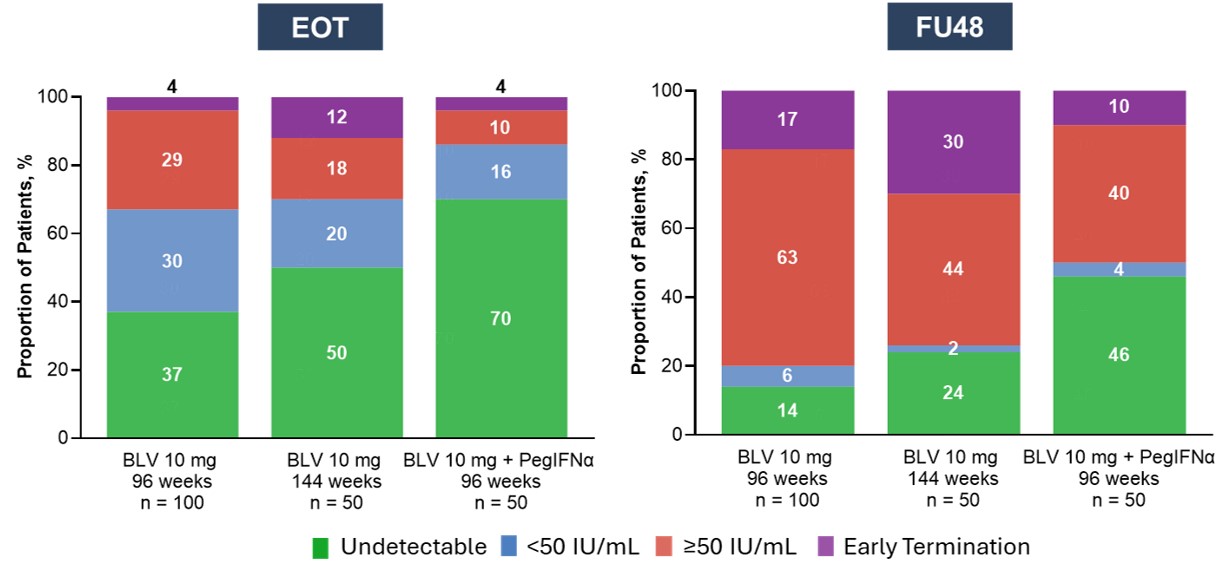
Figure: Table. Demographics and Baseline Disease Characteristics.
*PegIFNα + BLV 10 mg: n = 1, other race.
ALT, alanine aminotransferase; BLV, bulevirtide; HBV, hepatitis B virus; HDV, hepatitis delta virus; NA, nucleos(t)ide analogue; PegIFNα, pegylated interferon alpha.
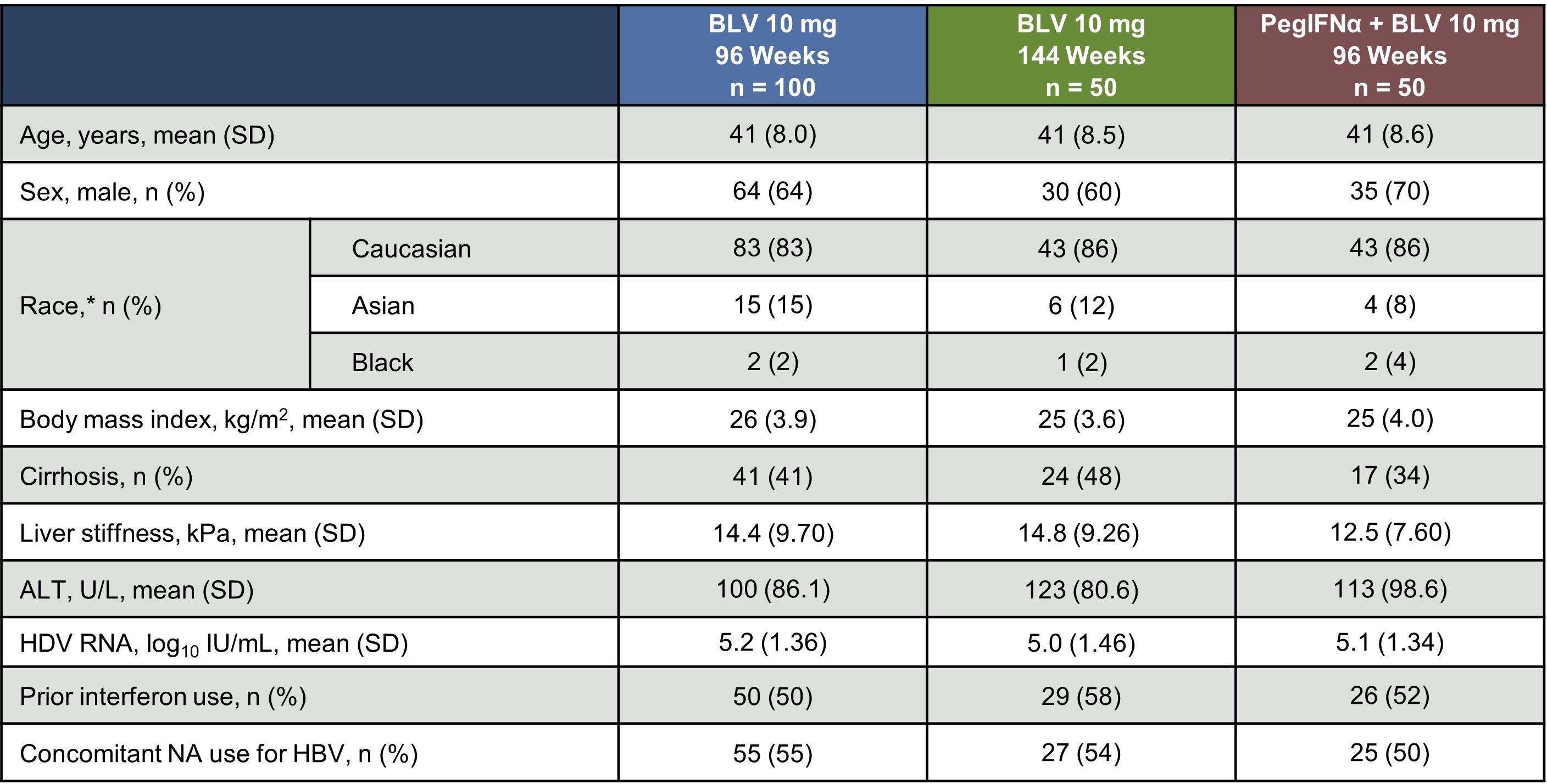
Figure: Figure. HDV RNA at EOT and FU48 Based on BLV Regimens.
Missing HDV RNA data not due to early termination were imputed as ≥50 IU/mL.
BLV, bulevirtide; EOT, end of treatment; FU48, follow-up at week 48 after EOT; HDV, hepatitis delta virus; PegIFNα, pegylated interferon alpha.
Disclosures:
Fabien Zoulim: Aligos Therapeutics – Consultant, Grant/Research Support. Assembly Biosciences – Consultant, Grant/Research Support. Gilead Sciences, Inc. – Consultant. GSK – Consultant. Vir Biotechnology – Consultant.
Tarik Asselah: AbbVie – Investigator, Speakers Bureau. Eiger Pharmaceuticals – Investigator, Speakers Bureau. Gilead Sciences, Inc. – Investigator, Speakers Bureau. Janssen – Investigator, Speakers Bureau. Merck – Investigator, Speakers Bureau. MYR Pharmaceuticals – Investigator, Speakers Bureau. Roche – Investigator, Speakers Bureau.
Soo Aleman: AbbVie – Honoraria for educational events, Speakers Bureau. Biogen – Honoraria for educational events, Speakers Bureau. Gilead Sciences, Inc. – Honoraria for educational events, Speakers Bureau. GSK – Honoraria for educational events, Speakers Bureau. Janssen – Honoraria for educational events, Speakers Bureau. Merck Sharp & Dohme – Honoraria for educational events, Speakers Bureau. MYR GmbH – Honoraria for educational events, Speakers Bureau. Roche – Honoraria for educational events, Speakers Bureau. Spring Bank Pharmaceuticals – Honoraria for educational events, Speakers Bureau.
Maurizia Brunetto: AbbVie – Consultant, Speakers Bureau. AstraZeneca – Consultant, Speakers Bureau. Gilead Sciences, Inc. – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Merck Sharp & Dohme-Eisai – Consultant, Speakers Bureau. Roche – Consultant, Speakers Bureau.
Vladimir Chulanov: AbbVie – Consultant, Speakers Bureau. AstraZeneca – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Gilead Sciences, Inc. – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hepatera – Consultant, Speakers Bureau. Merck Sharp & Dohme – Consultant, Speakers Bureau. Roche – Consultant, Speakers Bureau. R-Pharm – Consultant, Speakers Bureau.
Adrian Streinu-Cercel indicated no relevant financial relationships.
George Sebastian Gherlan indicated no relevant financial relationships.
Pavel Bogomolov: AbbVie – Grant/Research Support, Speakers Bureau. Bayer – Grant/Research Support, Speakers Bureau. Gilead Sciences, Inc. – Grant/Research Support, Speakers Bureau. Hepatera – Grant/Research Support, Speakers Bureau. Merck Sharp & Dohme – Grant/Research Support, Speakers Bureau. Norvo Nordisk – Grant/Research Support, Speakers Bureau. R-Pharm – Grant/Research Support, Speakers Bureau.
Tatiana Stepanova indicated no relevant financial relationships.
Viacheslav Morozov indicated no relevant financial relationships.
Olga Sagalova indicated no relevant financial relationships.
Renee-Claude Mercier: Gilead Sciences, Inc. – Employee, Stock Options.
Lei Ye: Gilead Sciences, Inc. – Employee, Stock Options.
Amos Lichtman: Gilead Sciences, Inc. – Employee, Stock Options.
Dmitry Manuilov: Gilead Sciences, Inc. – Employee, Stock Options.
Heiner Wedemeyer: Abbott Laboratories – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Abbott Molecular – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Albireo Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. AstraZeneca – Advisory Committee/Board Member, Consultant, Speakers Bureau. Atea Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. BioMarin Pharmaceutical – Advisory Committee/Board Member, Consultant, Speakers Bureau. Biotest – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. CSL Behring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Dr. Falk Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Falk Foundation – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead Sciences, Inc. – Advisory Committee/Board Member, Consultant, Speakers Bureau. GSK – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lilly Deutschland – Advisory Committee/Board Member, Consultant, Speakers Bureau. Merck Sharp & Dohme – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mirum Pharma Germany – Advisory Committee/Board Member, Consultant, Speakers Bureau. Olink – Advisory Committee/Board Member, Consultant, Speakers Bureau. Orphalan – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche Diagnostics International – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Swedish Orphan Biovitrum – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda Pharma Vertrieb – Advisory Committee/Board Member, Consultant, Speakers Bureau. Vir Biotechnology – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Pietro Lampertico: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Aligos Therapeutics – Advisory Committee/Board Member, Speakers Bureau. Alnylam Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau. Antios Therapeutics – Advisory Committee/Board Member, Speakers Bureau. Arrowhead Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Eiger Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau. Gilead Sciences, Inc. – Advisory Committee/Board Member, Speakers Bureau. GSK – Advisory Committee/Board Member, Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. Merck Sharp & Dohme – Advisory Committee/Board Member, Speakers Bureau. MYR GmbH – Advisory Committee/Board Member, Speakers Bureau. Roche – Advisory Committee/Board Member, Speakers Bureau. Spring Bank Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau.
Fabien Zoulim, MD, PhD1, Tarik Asselah, MD, PhD2, Soo Aleman, MD, PhD3, Maurizia R. Brunetto, MD4, Vladimir Chulanov, MD, PhD, DSc5, Adrian Streinu-Cercel, MD, PhD6, George Sebastian Gherlan, MD7, Pavel Bogomolov, MD, PhD8, Tatiana Stepanova, MD9, Viacheslav Morozov, MD, PhD10, Olga Sagalova, PhD11, Renee-Claude Mercier, PharmD12, Lei Ye, PhD12, Amos Lichtman, MD, MPH12, Dmitry Manuilov, MD12, Heiner Wedemeyer, MD13, Pietro Lampertico, MD, PhD14. P3780 - Achieving Undetectable Hepatitis Delta Virus RNA at End of Therapy With Bulevirtide 10 Mg/day With or Without PegIFNα Is Strongly Associated With Posttreatment Virologic Response in Chronic Hepatitis Delta, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Hospital Croix Rousse, Lyon Hepatology Institute, Lyon, Provence-Alpes-Cote d'Azur, France; 2Hôpital Beaujon APHP, Université de Paris, INSERM, Clichy, Ile-de-France, France; 3Department of Infectious Diseases, Karolinska University Hospital/Karolinska Institutet, Stockholm, Stockholms Lan, Sweden; 4Hepatology Unit, Reference Center of the Tuscany Region for Chronic Liver Disease and Cancer, University Hospital of Pisa; Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Toscana, Italy; 5Sechenov University, Moscow, Moskva, Russia; 6National Institute of Infectious Diseases Prof. Dr. Matei Bals; University of Medicine and Pharmacy “Carol Davila” Bucharest, Bucharest, Bucuresti, Romania; 7University of Medicine and Pharmacy “Carol Davila” Bucharest; Dr. Victor Babes Foundation, Bucharest, Bucuresti, Romania; 8M.F. Vladimirsky Moscow Regional Research and Clinical Institute, Moscow, Moskva, Russia; 9LLC Clinic of Modern Medicine, Moscow, Moskva, Russia; 10LLC Medical Company “Hepatolog”, Samara, Samara, Russia; 11South Ural State Medical University, Chelyabinsk, Orel, Russia; 12Gilead Sciences, Inc., Foster City, CA; 13Clinic for Gastroenterology, Hepatology, Infectious Diseases, and Endocrinology, Hannover Medical School, Hannover, Sachsen, Germany; 14Division of Gastroenterology and Hepatology, Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico; CRC “A. M. and A. Migliavacca” Center for Liver Disease, Department of Pathophysiology and Transplantation, University of Milan, Milan, Lombardia, Italy
Introduction: Bulevirtide (BLV) 2 mg is approved for chronic hepatitis delta (CHD) treatment in Europe, Australia, and Russia. In an integrated analysis of BLV 10 mg monotherapy or in combination with pegylated interferon alpha (PegIFNα), we explored whether hepatitis delta virus (HDV) RNA levels undetectable (target not detected; TND) vs below the lower limit of quantitation (< LLOQ; target detected [TD]) at end of treatment (EOT) affect posttreatment (PT) virologic response.
Methods: A pooled analysis of data was performed from patients (pts) who completed 2 or 3 years of BLV 10 mg/day (d) with or without PegIFNα in the MYR204 Phase 2 and MYR301 Phase 3 studies. Pts received (A) BLV 10 mg + PegIFNα for 48 weeks (W) followed by 48W of monotherapy with BLV 10 mg (n = 50), (B) BLV 10 mg for 96W (n = 100), or (C) BLV 10 mg for 144W (n = 50). Pts were followed for up to 48W after EOT (follow-up [FU]48). HDV RNA levels were determined by RT-qPCR using RoboGene 2.0 (LLOQ 50 IU/mL, limit of detection 6 IU/mL). Virologic response rates at FU48 were compared between pts with undetectable HDV RNA and those with HDV RNA < LLOQ, TD at EOT.
Results: Demographics were similar across groups (Table). At EOT, 49% (97/200) achieved undetectable HDV RNA: (A) 70% (35/50), (B) 37% (37/100), and (C) 50% (25/50); 24% (48/200) had < LLOQ, TD: (A) 16% (8/50), (B) 30% (30/100), and (C) 20% (10/50; Figure). At FU48, undetectable HDV RNA was observed in 25% (49/200) overall: (A) 23/50 (46%) of pts receiving combination therapy, (B) 14% (14/100) of pts receiving 2 years of BLV monotherapy, and (C) 24% (12/50) of pts receiving 3 years of BLV monotherapy. Of the pts with undetectable HDV RNA at EOT, 46% (45/97) maintained undetectable HDV RNA at FU48: (A) 60% (21/35), (B) 35% (13/37), and (C) 44% (11/25); 6% (6/97) had < LLOQ, TD at FU48: (A) 6% (2/35), (B) 9% (3/37), and (C) 4% (1/25). Of those who had < LLOQ, TD at EOT, only 6% (3/48) had undetectable HDV RNA (1 per group) and 4% (2/48) remained < LLOQ, TD at FU48, while 71% (34/48) had HDV RNA >LLOQ: (A) 62.5% (5/8), (B) 80% (24/30), and (C) 50% (5/10); during FU, 9 discontinued the study early.
Discussion: In pts with compensated CHD, achieving undetectable HDV RNA with TND at EOT is associated with virologic suppression at 48W PT. Combination therapy with BLV 10 mg/d and PegIFNα and longer treatment with BLV monotherapy were associated with higher rates of undetectable HDV RNA at EOT and FU48. Pts with HDV RNA < LLOQ, TD at EOT are likely to have HDV RNA rebound off therapy.

Figure: Table. Demographics and Baseline Disease Characteristics.
*PegIFNα + BLV 10 mg: n = 1, other race.
ALT, alanine aminotransferase; BLV, bulevirtide; HBV, hepatitis B virus; HDV, hepatitis delta virus; NA, nucleos(t)ide analogue; PegIFNα, pegylated interferon alpha.

Figure: Figure. HDV RNA at EOT and FU48 Based on BLV Regimens.
Missing HDV RNA data not due to early termination were imputed as ≥50 IU/mL.
BLV, bulevirtide; EOT, end of treatment; FU48, follow-up at week 48 after EOT; HDV, hepatitis delta virus; PegIFNα, pegylated interferon alpha.
Disclosures:
Fabien Zoulim: Aligos Therapeutics – Consultant, Grant/Research Support. Assembly Biosciences – Consultant, Grant/Research Support. Gilead Sciences, Inc. – Consultant. GSK – Consultant. Vir Biotechnology – Consultant.
Tarik Asselah: AbbVie – Investigator, Speakers Bureau. Eiger Pharmaceuticals – Investigator, Speakers Bureau. Gilead Sciences, Inc. – Investigator, Speakers Bureau. Janssen – Investigator, Speakers Bureau. Merck – Investigator, Speakers Bureau. MYR Pharmaceuticals – Investigator, Speakers Bureau. Roche – Investigator, Speakers Bureau.
Soo Aleman: AbbVie – Honoraria for educational events, Speakers Bureau. Biogen – Honoraria for educational events, Speakers Bureau. Gilead Sciences, Inc. – Honoraria for educational events, Speakers Bureau. GSK – Honoraria for educational events, Speakers Bureau. Janssen – Honoraria for educational events, Speakers Bureau. Merck Sharp & Dohme – Honoraria for educational events, Speakers Bureau. MYR GmbH – Honoraria for educational events, Speakers Bureau. Roche – Honoraria for educational events, Speakers Bureau. Spring Bank Pharmaceuticals – Honoraria for educational events, Speakers Bureau.
Maurizia Brunetto: AbbVie – Consultant, Speakers Bureau. AstraZeneca – Consultant, Speakers Bureau. Gilead Sciences, Inc. – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Merck Sharp & Dohme-Eisai – Consultant, Speakers Bureau. Roche – Consultant, Speakers Bureau.
Vladimir Chulanov: AbbVie – Consultant, Speakers Bureau. AstraZeneca – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Gilead Sciences, Inc. – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hepatera – Consultant, Speakers Bureau. Merck Sharp & Dohme – Consultant, Speakers Bureau. Roche – Consultant, Speakers Bureau. R-Pharm – Consultant, Speakers Bureau.
Adrian Streinu-Cercel indicated no relevant financial relationships.
George Sebastian Gherlan indicated no relevant financial relationships.
Pavel Bogomolov: AbbVie – Grant/Research Support, Speakers Bureau. Bayer – Grant/Research Support, Speakers Bureau. Gilead Sciences, Inc. – Grant/Research Support, Speakers Bureau. Hepatera – Grant/Research Support, Speakers Bureau. Merck Sharp & Dohme – Grant/Research Support, Speakers Bureau. Norvo Nordisk – Grant/Research Support, Speakers Bureau. R-Pharm – Grant/Research Support, Speakers Bureau.
Tatiana Stepanova indicated no relevant financial relationships.
Viacheslav Morozov indicated no relevant financial relationships.
Olga Sagalova indicated no relevant financial relationships.
Renee-Claude Mercier: Gilead Sciences, Inc. – Employee, Stock Options.
Lei Ye: Gilead Sciences, Inc. – Employee, Stock Options.
Amos Lichtman: Gilead Sciences, Inc. – Employee, Stock Options.
Dmitry Manuilov: Gilead Sciences, Inc. – Employee, Stock Options.
Heiner Wedemeyer: Abbott Laboratories – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Abbott Molecular – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Albireo Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. AstraZeneca – Advisory Committee/Board Member, Consultant, Speakers Bureau. Atea Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. BioMarin Pharmaceutical – Advisory Committee/Board Member, Consultant, Speakers Bureau. Biotest – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. CSL Behring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Dr. Falk Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Falk Foundation – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead Sciences, Inc. – Advisory Committee/Board Member, Consultant, Speakers Bureau. GSK – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lilly Deutschland – Advisory Committee/Board Member, Consultant, Speakers Bureau. Merck Sharp & Dohme – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mirum Pharma Germany – Advisory Committee/Board Member, Consultant, Speakers Bureau. Olink – Advisory Committee/Board Member, Consultant, Speakers Bureau. Orphalan – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche Diagnostics International – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Swedish Orphan Biovitrum – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda Pharma Vertrieb – Advisory Committee/Board Member, Consultant, Speakers Bureau. Vir Biotechnology – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Pietro Lampertico: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Aligos Therapeutics – Advisory Committee/Board Member, Speakers Bureau. Alnylam Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau. Antios Therapeutics – Advisory Committee/Board Member, Speakers Bureau. Arrowhead Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Eiger Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau. Gilead Sciences, Inc. – Advisory Committee/Board Member, Speakers Bureau. GSK – Advisory Committee/Board Member, Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. Merck Sharp & Dohme – Advisory Committee/Board Member, Speakers Bureau. MYR GmbH – Advisory Committee/Board Member, Speakers Bureau. Roche – Advisory Committee/Board Member, Speakers Bureau. Spring Bank Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau.
Fabien Zoulim, MD, PhD1, Tarik Asselah, MD, PhD2, Soo Aleman, MD, PhD3, Maurizia R. Brunetto, MD4, Vladimir Chulanov, MD, PhD, DSc5, Adrian Streinu-Cercel, MD, PhD6, George Sebastian Gherlan, MD7, Pavel Bogomolov, MD, PhD8, Tatiana Stepanova, MD9, Viacheslav Morozov, MD, PhD10, Olga Sagalova, PhD11, Renee-Claude Mercier, PharmD12, Lei Ye, PhD12, Amos Lichtman, MD, MPH12, Dmitry Manuilov, MD12, Heiner Wedemeyer, MD13, Pietro Lampertico, MD, PhD14. P3780 - Achieving Undetectable Hepatitis Delta Virus RNA at End of Therapy With Bulevirtide 10 Mg/day With or Without PegIFNα Is Strongly Associated With Posttreatment Virologic Response in Chronic Hepatitis Delta, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
