Monday Poster Session
Category: Liver
P3855 - Bicalutamide: A Rare Cause of Drug-Induced Autoimmune Hepatitis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- ED
Erika M. Dorff, MD, MPH (she/her/hers)
University of Vermont Medical Center
Burlington, VT
Presenting Author(s)
Erika M. Dorff, MD, MPH, Aurasch Moaven, MD, Natalia Liu, MD, Nicholas Ferrentino, MD
University of Vermont Medical Center, Burlington, VT
Introduction: Bicalutamide is an oral, nonsteroidal antiandrogen medication that is used to treat prostate cancer. It has been associated with mild, transient transaminase elevations and cases of self-limiting drug-induced liver injury (DILI). Drug-induced autoimmune hepatitis (DIAIH) is a rare type of DILI and is typically associated with antibiotics. Diagnostic criteria are similar to that of idiopathic autoimmune hepatitis (AIH), but histologic findings of DIAIH often include features of both DILI and AIH. In this report, we present a case of bicalutamide-induced DIAIH.
Case Description/
Methods: A 68-year-old male with history of prostate adenocarcinoma undergoing radiation therapy with concurrent bicalutamide presented with lightheadedness and fatigue approximately two months after starting treatment. Labs were concerning for acute severe liver injury including alanine aminotransferase (ALT) of 1783, aspartate aminotransferase (AST) of 1179, total bilirubin of 4.9, and alkaline phosphatase of 542. He had no laboratory evidence of hepatic synthetic dysfunction. Imaging demonstrated nonspecific edematous gallbladder wall thickening and periportal edema consistent with acute hepatitis. Viral studies, acetaminophen level, and infectious workup were negative. Labs also showed an IgG of 2,935 and anti-nuclear antibody (ANA) >1:640 with negative anti-smooth muscle antibody. Liver biopsy demonstrated eosinophils and plasma cells with interface hepatitis, consistent with DIAIH. Bicalutamide was discontinued and he was discharged on a prednisone taper. He demonstrated improvement in liver function tests in weeks following discharge.
Discussion: To date, there have been no reports of bicalutamide associated DIAIH and cases of autoantibody formation following initiation of bicalutamide has not been described in the literature. DIAIH typically takes months to years to develop and recovery is delayed, often leading to use of steroid therapy to completely resolve the injury as was the case in this patient. In some cases, patients require additional immunosuppressive therapy, such as azathioprine, to lead to complete resolution in DIAIH. There have been reports of other nonsteroidal antiandrogens leading to DILI and even acute liver failure. Bicalutamide has not been associated with acute liver failure, though it has been used less extensively compared to other antiandrogen therapies. Further research is warranted to determine long term hepatic effects and outcomes of DIAIH associated with bicalutamide.
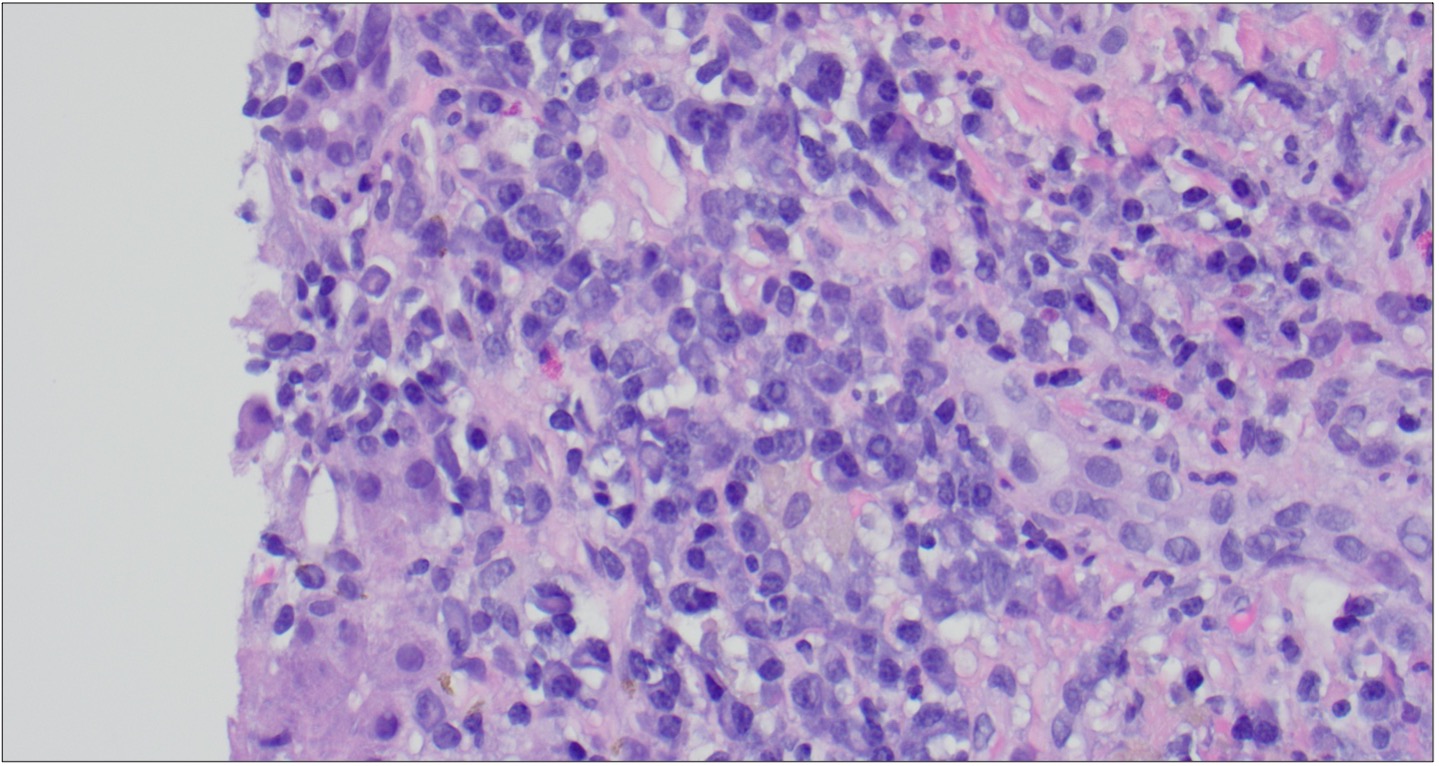
Figure: Figure 1. At left, photomicrograph of liver core biopsy showing sheets and clusters of plasma cells and occasional eosinophils and adjacent areas of parenchymal collapse (Hematoxylin and eosin, 10x). At right, photomicrograph of liver core biopsy showing interface activity composed of plasma cells clusters and occasional eosinophils (Hematoxylin and eosin, 20x).
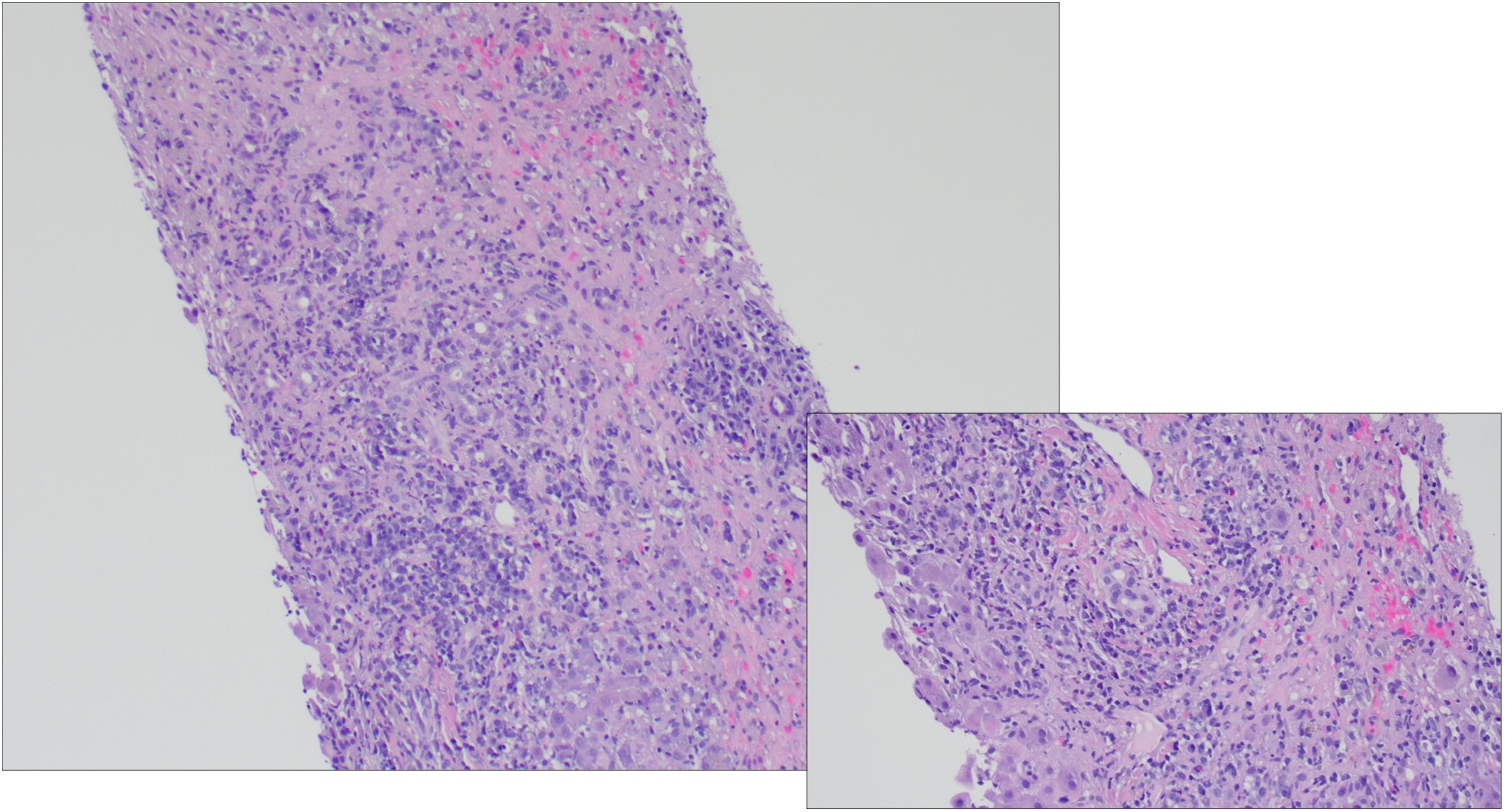
Figure: Figure 2. Photomicrograph of liver core biopsy showing plasma cell clusters (Hematoxylin and eosin, 40x).
Disclosures:
Erika Dorff indicated no relevant financial relationships.
Aurasch Moaven indicated no relevant financial relationships.
Natalia Liu indicated no relevant financial relationships.
Nicholas Ferrentino indicated no relevant financial relationships.
Erika M. Dorff, MD, MPH, Aurasch Moaven, MD, Natalia Liu, MD, Nicholas Ferrentino, MD. P3855 - Bicalutamide: A Rare Cause of Drug-Induced Autoimmune Hepatitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
University of Vermont Medical Center, Burlington, VT
Introduction: Bicalutamide is an oral, nonsteroidal antiandrogen medication that is used to treat prostate cancer. It has been associated with mild, transient transaminase elevations and cases of self-limiting drug-induced liver injury (DILI). Drug-induced autoimmune hepatitis (DIAIH) is a rare type of DILI and is typically associated with antibiotics. Diagnostic criteria are similar to that of idiopathic autoimmune hepatitis (AIH), but histologic findings of DIAIH often include features of both DILI and AIH. In this report, we present a case of bicalutamide-induced DIAIH.
Case Description/
Methods: A 68-year-old male with history of prostate adenocarcinoma undergoing radiation therapy with concurrent bicalutamide presented with lightheadedness and fatigue approximately two months after starting treatment. Labs were concerning for acute severe liver injury including alanine aminotransferase (ALT) of 1783, aspartate aminotransferase (AST) of 1179, total bilirubin of 4.9, and alkaline phosphatase of 542. He had no laboratory evidence of hepatic synthetic dysfunction. Imaging demonstrated nonspecific edematous gallbladder wall thickening and periportal edema consistent with acute hepatitis. Viral studies, acetaminophen level, and infectious workup were negative. Labs also showed an IgG of 2,935 and anti-nuclear antibody (ANA) >1:640 with negative anti-smooth muscle antibody. Liver biopsy demonstrated eosinophils and plasma cells with interface hepatitis, consistent with DIAIH. Bicalutamide was discontinued and he was discharged on a prednisone taper. He demonstrated improvement in liver function tests in weeks following discharge.
Discussion: To date, there have been no reports of bicalutamide associated DIAIH and cases of autoantibody formation following initiation of bicalutamide has not been described in the literature. DIAIH typically takes months to years to develop and recovery is delayed, often leading to use of steroid therapy to completely resolve the injury as was the case in this patient. In some cases, patients require additional immunosuppressive therapy, such as azathioprine, to lead to complete resolution in DIAIH. There have been reports of other nonsteroidal antiandrogens leading to DILI and even acute liver failure. Bicalutamide has not been associated with acute liver failure, though it has been used less extensively compared to other antiandrogen therapies. Further research is warranted to determine long term hepatic effects and outcomes of DIAIH associated with bicalutamide.

Figure: Figure 1. At left, photomicrograph of liver core biopsy showing sheets and clusters of plasma cells and occasional eosinophils and adjacent areas of parenchymal collapse (Hematoxylin and eosin, 10x). At right, photomicrograph of liver core biopsy showing interface activity composed of plasma cells clusters and occasional eosinophils (Hematoxylin and eosin, 20x).

Figure: Figure 2. Photomicrograph of liver core biopsy showing plasma cell clusters (Hematoxylin and eosin, 40x).
Disclosures:
Erika Dorff indicated no relevant financial relationships.
Aurasch Moaven indicated no relevant financial relationships.
Natalia Liu indicated no relevant financial relationships.
Nicholas Ferrentino indicated no relevant financial relationships.
Erika M. Dorff, MD, MPH, Aurasch Moaven, MD, Natalia Liu, MD, Nicholas Ferrentino, MD. P3855 - Bicalutamide: A Rare Cause of Drug-Induced Autoimmune Hepatitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

