Monday Poster Session
Category: Small Intestine
P4020 - Risk of Incident Celiac Disease in Cancer Patients Treated With Immune Checkpoint Inhibitors: A Large Propensity-Matched Cohort Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Danah Al-Deiri, MD (she/her/hers)
Cleveland Clinic Foundation
Cleveland, OH
Presenting Author(s)
Danah Al-Deiri, MD1, Hussam Kawas, MD1, Sara Haddad, MD2, Julia Wajsberg, MD3, Joseph Sleiman, MD1, Claire Jansson-Knodell, MD1, Jessica R. Philpott, MD, PhD, FACG1
1Cleveland Clinic Foundation, Cleveland, OH; 2Cleveland Clinic, Cleveland, OH; 3University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH
Introduction: Immune checkpoint inhibitors (ICIs) have transformed cancer treatment but are associated with immune-related adverse events (irAEs). While gastrointestinal irAEs such as colitis are well recognized, the potential for ICIs to trigger de novo celiac disease, a small intestinal immune-mediated condition, remains poorly studied. We aimed to assess the risk of developing incident celiac disease coding in cancer patients receiving ICIs compared to those not treated with ICIs.
Methods: Using the TriNetX real-world database, we conducted a retrospective cohort study of adult cancer patients treated between March 2011 and January 2024. Patients were categorized into ICI-exposed and non-exposed cohorts. Those with pre-existing celiac disease codes were excluded. The primary outcome was new coding for celiac disease within one year of cancer treatment initiation. Propensity score matching (1:1) was performed for age, sex, demographics, and cancer type. A p-value < 0.05 was considered statistically significant.
Results: We identified 65,245 ICI-exposed and 3,533,194 non-exposed patients. After matching, each cohort included 35,668 patients. Groups were balanced by age (mean 63.2 vs 62.3 years) and sex (44.2% vs 45.1% female). Within one year, 22 patients (0.06%) in the ICI group had new celiac disease coding, compared to fewer than 10 patients (0.02%) in the non-exposed group. ICI exposure was associated with a significantly increased risk of incident celiac disease coding (RR 2.2, 95% CI 1.042–4.645; p=0.03).
Discussion: In this large, matched cohort study, ICI therapy was associated with more than a twofold increased risk of new celiac disease coding. Although incidence was low, this association warrants clinical attention. These findings underscore the need for heightened awareness of small intestinal irAEs and call for future studies to confirm true diagnoses of celiac disease and understand underlying mechanisms of ICI-associated enteropathy.
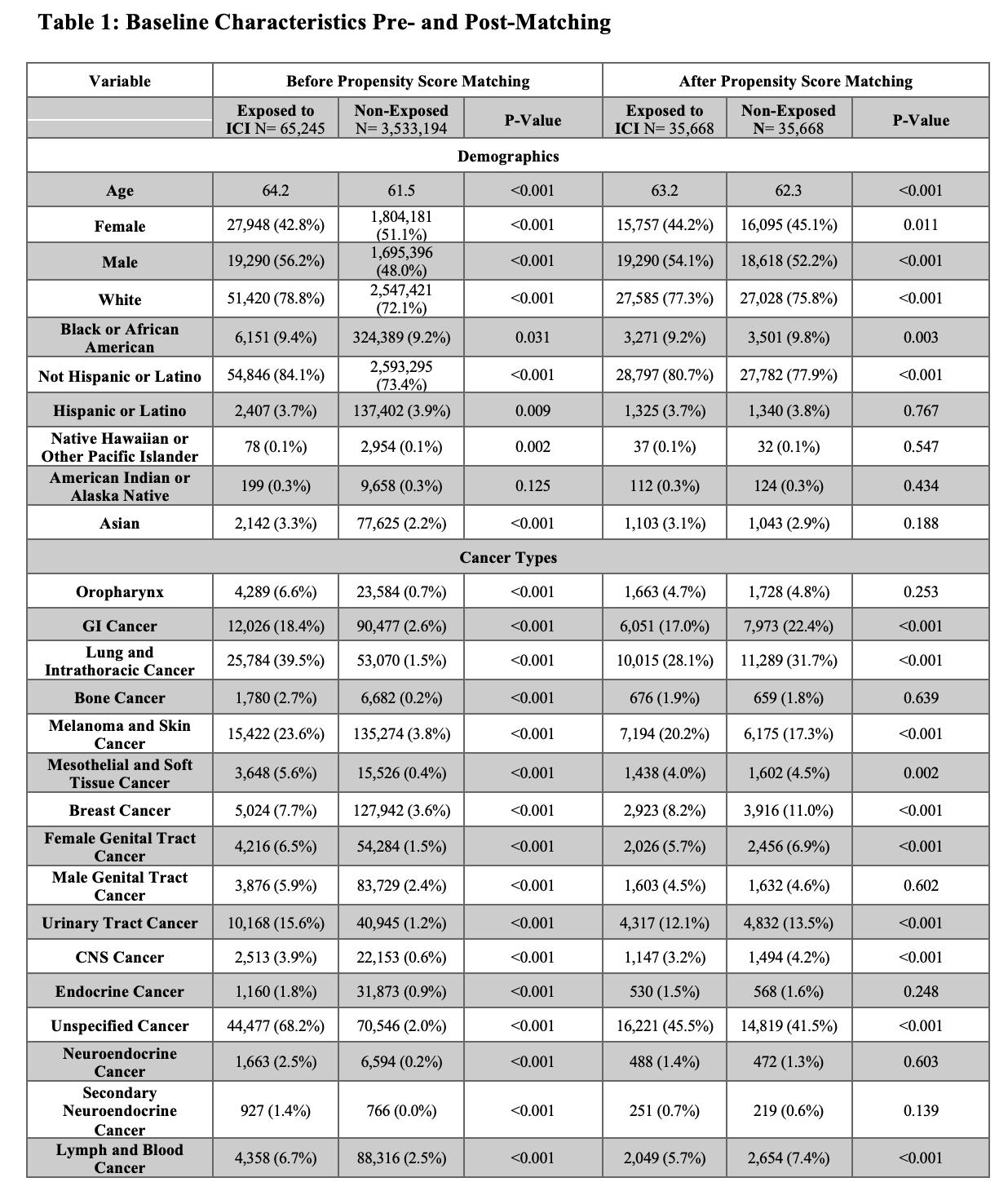
Figure: Comparison of baseline demographics and cancer diagnoses between study groups before and after propensity score matching
Disclosures:
Danah Al-Deiri indicated no relevant financial relationships.
Hussam Kawas indicated no relevant financial relationships.
Sara Haddad indicated no relevant financial relationships.
Julia Wajsberg indicated no relevant financial relationships.
Joseph Sleiman indicated no relevant financial relationships.
Claire Jansson-Knodell: AGA-Takeda Pharmaceuticals – Grant/Research Support. Exact Sciences – Stock-publicly held company(excluding mutual/index funds). Johnson&Johnson – Stock-publicly held company(excluding mutual/index funds). Medtronic – Stock-publicly held company(excluding mutual/index funds). United Health Group – Stock-publicly held company(excluding mutual/index funds).
Jessica Philpott: Abbvie – Speakers Bureau.
Danah Al-Deiri, MD1, Hussam Kawas, MD1, Sara Haddad, MD2, Julia Wajsberg, MD3, Joseph Sleiman, MD1, Claire Jansson-Knodell, MD1, Jessica R. Philpott, MD, PhD, FACG1. P4020 - Risk of Incident Celiac Disease in Cancer Patients Treated With Immune Checkpoint Inhibitors: A Large Propensity-Matched Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Cleveland Clinic Foundation, Cleveland, OH; 2Cleveland Clinic, Cleveland, OH; 3University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH
Introduction: Immune checkpoint inhibitors (ICIs) have transformed cancer treatment but are associated with immune-related adverse events (irAEs). While gastrointestinal irAEs such as colitis are well recognized, the potential for ICIs to trigger de novo celiac disease, a small intestinal immune-mediated condition, remains poorly studied. We aimed to assess the risk of developing incident celiac disease coding in cancer patients receiving ICIs compared to those not treated with ICIs.
Methods: Using the TriNetX real-world database, we conducted a retrospective cohort study of adult cancer patients treated between March 2011 and January 2024. Patients were categorized into ICI-exposed and non-exposed cohorts. Those with pre-existing celiac disease codes were excluded. The primary outcome was new coding for celiac disease within one year of cancer treatment initiation. Propensity score matching (1:1) was performed for age, sex, demographics, and cancer type. A p-value < 0.05 was considered statistically significant.
Results: We identified 65,245 ICI-exposed and 3,533,194 non-exposed patients. After matching, each cohort included 35,668 patients. Groups were balanced by age (mean 63.2 vs 62.3 years) and sex (44.2% vs 45.1% female). Within one year, 22 patients (0.06%) in the ICI group had new celiac disease coding, compared to fewer than 10 patients (0.02%) in the non-exposed group. ICI exposure was associated with a significantly increased risk of incident celiac disease coding (RR 2.2, 95% CI 1.042–4.645; p=0.03).
Discussion: In this large, matched cohort study, ICI therapy was associated with more than a twofold increased risk of new celiac disease coding. Although incidence was low, this association warrants clinical attention. These findings underscore the need for heightened awareness of small intestinal irAEs and call for future studies to confirm true diagnoses of celiac disease and understand underlying mechanisms of ICI-associated enteropathy.

Figure: Comparison of baseline demographics and cancer diagnoses between study groups before and after propensity score matching
Disclosures:
Danah Al-Deiri indicated no relevant financial relationships.
Hussam Kawas indicated no relevant financial relationships.
Sara Haddad indicated no relevant financial relationships.
Julia Wajsberg indicated no relevant financial relationships.
Joseph Sleiman indicated no relevant financial relationships.
Claire Jansson-Knodell: AGA-Takeda Pharmaceuticals – Grant/Research Support. Exact Sciences – Stock-publicly held company(excluding mutual/index funds). Johnson&Johnson – Stock-publicly held company(excluding mutual/index funds). Medtronic – Stock-publicly held company(excluding mutual/index funds). United Health Group – Stock-publicly held company(excluding mutual/index funds).
Jessica Philpott: Abbvie – Speakers Bureau.
Danah Al-Deiri, MD1, Hussam Kawas, MD1, Sara Haddad, MD2, Julia Wajsberg, MD3, Joseph Sleiman, MD1, Claire Jansson-Knodell, MD1, Jessica R. Philpott, MD, PhD, FACG1. P4020 - Risk of Incident Celiac Disease in Cancer Patients Treated With Immune Checkpoint Inhibitors: A Large Propensity-Matched Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
