Monday Poster Session
Category: Liver
P4018 - Fibrillating Liver: Effective Treatment of Amiodarone-Induced Acute Liver Injury - A Case Report
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Ahmad Zain, MBBS (he/him/his)
Parkview Medical Center
Pueblo, CO
Presenting Author(s)
Ahmad Zain, MBBS1, Hammad Qadri, DO2, Fatima Ashfaq, MBBS3, Amil Shah, DO1, Osama Ijaz, MD4, Muhammad Sohaib, MBBS1, Syed Rafay H zaidi, MBBS1, Mustafa Nayeem, MD1
1Parkview Medical Center, Pueblo, CO; 2United Health Services, Wilson Medical Center, Vestal, NY; 3Nishtar Medical University, Multan, Punjab, Pakistan; 4SSM Health St. Mary's Hospital - St. Louis, Richmond Heights, MO
Introduction: In the USA, drug-induced liver injury is the leading cause of acute liver failure. Amiodarone, a widely used antiarrhythmic medication rarely causes acute liver injury when administered intravenously(IV). Studies suggest that polysorbate 80, a solvent for amiodarone, may directly harm hepatocytes, rather than the drug itself. We present a rare case of acute liver injury in an elderly patient shortly after initiating IV amiodarone.
Case Description/
Methods: A 94-year-old female with a history of coronary artery disease, atrial fibrillation, dyslipidemia, presenting with acute onset right lower extremity pain. She was diagnosed with acute right superficial femoral artery occlusion and underwent emergent open thrombectomy and balloon angioplasty. Amiodarone was initiated for atrial fibrillation, starting with a 150 mg bolus followed by a 360 mg infusion over 6 hours. Twelve hours later, liver function tests (LFTs) showed severe elevations in ALT, AST, and bilirubin levels in hepatocellular pattern despite prior normal levels as trends shown in Table 1 Amiodarone was immediately discontinued and metoprolol succinate was used for rate control. She was hemodynamically stable without any evidence of hypotension and Liver disease workup was negative. LFTs normalized at follow-up.
Discussion: Intravenous amiodarone is used in the short-term management of several arrhythmias and is primarily eliminated via biliary excretion. Acute hepatitis due to IV amiodarone is an extremely rare side effect and has been reported as early as 24 hours after the start of the drug with proposed mechanisms through ischemic hepatic injury secondary to relative hypotension, hypersensitivity reactions, vehicle-related toxicity (polysorbate-80), and idiosyncratic toxicity . In these cases, amiodarone should be discontinued immediately.In our case, alternative potential diagnoses, such as viral hepatitis, autoimmune hepatitis, and acetaminophen toxicity, were ruled out based on negative laboratory findings. The literature commonly discusses ischemia as a primary cause of liver injury in acute scenarios though it was clinically excluded in our case Notably, there was no evidence of hypotension during the initial 48 hours prior to injury, ruling out ischemia. Additionally, recovery achieved through discontinuing amiodarone further reinforced the suspicion of drug-induced injury.
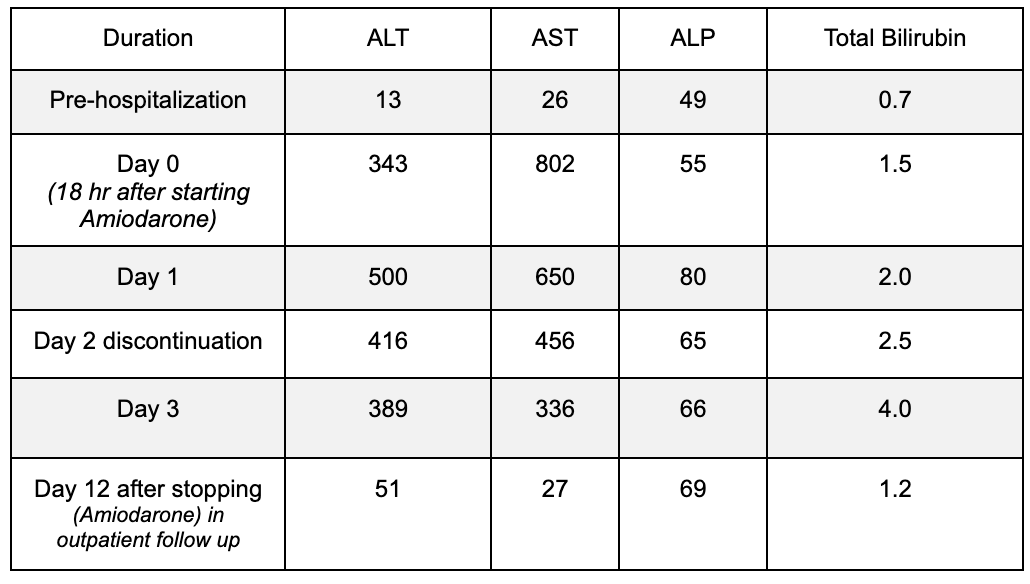
Figure: Table 1
Disclosures:
Ahmad Zain indicated no relevant financial relationships.
Hammad Qadri indicated no relevant financial relationships.
Fatima Ashfaq indicated no relevant financial relationships.
Amil Shah indicated no relevant financial relationships.
Osama Ijaz indicated no relevant financial relationships.
Muhammad Sohaib indicated no relevant financial relationships.
Syed Rafay H zaidi indicated no relevant financial relationships.
Mustafa Nayeem indicated no relevant financial relationships.
Ahmad Zain, MBBS1, Hammad Qadri, DO2, Fatima Ashfaq, MBBS3, Amil Shah, DO1, Osama Ijaz, MD4, Muhammad Sohaib, MBBS1, Syed Rafay H zaidi, MBBS1, Mustafa Nayeem, MD1. P4018 - Fibrillating Liver: Effective Treatment of Amiodarone-Induced Acute Liver Injury - A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Parkview Medical Center, Pueblo, CO; 2United Health Services, Wilson Medical Center, Vestal, NY; 3Nishtar Medical University, Multan, Punjab, Pakistan; 4SSM Health St. Mary's Hospital - St. Louis, Richmond Heights, MO
Introduction: In the USA, drug-induced liver injury is the leading cause of acute liver failure. Amiodarone, a widely used antiarrhythmic medication rarely causes acute liver injury when administered intravenously(IV). Studies suggest that polysorbate 80, a solvent for amiodarone, may directly harm hepatocytes, rather than the drug itself. We present a rare case of acute liver injury in an elderly patient shortly after initiating IV amiodarone.
Case Description/
Methods: A 94-year-old female with a history of coronary artery disease, atrial fibrillation, dyslipidemia, presenting with acute onset right lower extremity pain. She was diagnosed with acute right superficial femoral artery occlusion and underwent emergent open thrombectomy and balloon angioplasty. Amiodarone was initiated for atrial fibrillation, starting with a 150 mg bolus followed by a 360 mg infusion over 6 hours. Twelve hours later, liver function tests (LFTs) showed severe elevations in ALT, AST, and bilirubin levels in hepatocellular pattern despite prior normal levels as trends shown in Table 1 Amiodarone was immediately discontinued and metoprolol succinate was used for rate control. She was hemodynamically stable without any evidence of hypotension and Liver disease workup was negative. LFTs normalized at follow-up.
Discussion: Intravenous amiodarone is used in the short-term management of several arrhythmias and is primarily eliminated via biliary excretion. Acute hepatitis due to IV amiodarone is an extremely rare side effect and has been reported as early as 24 hours after the start of the drug with proposed mechanisms through ischemic hepatic injury secondary to relative hypotension, hypersensitivity reactions, vehicle-related toxicity (polysorbate-80), and idiosyncratic toxicity . In these cases, amiodarone should be discontinued immediately.In our case, alternative potential diagnoses, such as viral hepatitis, autoimmune hepatitis, and acetaminophen toxicity, were ruled out based on negative laboratory findings. The literature commonly discusses ischemia as a primary cause of liver injury in acute scenarios though it was clinically excluded in our case Notably, there was no evidence of hypotension during the initial 48 hours prior to injury, ruling out ischemia. Additionally, recovery achieved through discontinuing amiodarone further reinforced the suspicion of drug-induced injury.

Figure: Table 1
Disclosures:
Ahmad Zain indicated no relevant financial relationships.
Hammad Qadri indicated no relevant financial relationships.
Fatima Ashfaq indicated no relevant financial relationships.
Amil Shah indicated no relevant financial relationships.
Osama Ijaz indicated no relevant financial relationships.
Muhammad Sohaib indicated no relevant financial relationships.
Syed Rafay H zaidi indicated no relevant financial relationships.
Mustafa Nayeem indicated no relevant financial relationships.
Ahmad Zain, MBBS1, Hammad Qadri, DO2, Fatima Ashfaq, MBBS3, Amil Shah, DO1, Osama Ijaz, MD4, Muhammad Sohaib, MBBS1, Syed Rafay H zaidi, MBBS1, Mustafa Nayeem, MD1. P4018 - Fibrillating Liver: Effective Treatment of Amiodarone-Induced Acute Liver Injury - A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
