Monday Poster Session
Category: Small Intestine
P4146 - Vedolizumab for Severe Refractory Acute Cellular Rejection After Intestinal Transplantation
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Michael Gianarakis, MD
University of Illinois at Chicago
Chicago, IL
Presenting Author(s)
Award: ACG Presidential Poster Award
Michael Gianarakis, MD1, Robert E. Carroll, MD2
1University of Illinois at Chicago, Chicago, IL; 2University of Illinois at Chicago, Department of Gastroenterology and Hepatology, Chicago, IL
Introduction: Despite advances in immunotherapy, acute cellular rejection (ACR) affects 30–40% of adult intestinal transplant (ITx) recipients. ACR is traditionally managed with high-dose corticosteroids and intensified immunosuppression. With the development of monoclonal antibodies targeting CD3, CD20, CD52, IL-2, and TNF-alpha, biologics are increasingly used for induction, maintenance, and anti-rejection therapy. Vedolizumab (VDZ), an anti-integrin α4β7 monoclonal antibody approved for inflammatory bowel disease (IBD), has gut-specific immunomodulatory effects that may offer a therapeutic option in intestinal graft rejection. We present a case of severe, treatment-refractory ACR successfully managed with VDZ.
Case Description/
Methods: A 19-year-old male developed short bowel syndrome after massive intestinal resection due to an abdominal gunshot wound and subsequently underwent ITx, including small intestine and ascending colon. He was continued on teduglutide, a glucagon-like peptide 2 analog (GLP-2) post-transplant and presented for diverting loop ileostomy reversal four months post-ITx. Surgical pathology showed mild ACR, for which infliximab and high-dose methylprednisolone were given. He was discharged on a prednisone taper with ongoing tacrolimus and monthly basiliximab. Two days later, he returned with abdominal pain and distension due to small bowel obstruction. He underwent limited small bowel resection with anastomosis and cecostomy creation. Endoscopy revealed denuded, edematous and ulcerated duodenal and colonic graft mucosa, and histology confirmed severe ACR. He received IV VDZ 300 mg, eculizumab 900 mg, and methylprednisolone 500 mg. Within four days of VDZ, serial endoscopies showed marked improvement in mucosal edema and inflammation with re-epithelialization (Figure 1). The graft continues to show endoscopic and histologic recovery following two doses of VDZ for ACR.
Discussion: This case highlights VDZ as a safe and effective treatment option for ACR in ITx. This is the third reported case of VDZ used for ACR in ITx and the first in a non-IBD patient. Our patient responded rapidly to VDZ after failing high-dose methylprednisolone, infliximab and basiliximab. VDZ’s gut-selective mechanism may allow for targeted immunosuppression with fewer systemic effects. Additionally, we postulate that GLP-2 analogs used in the post-transplant period may aid epithelial protection and mucosal recovery during ACR. Further studies are needed to assess long-term efficacy and safety of VDZ in this setting.
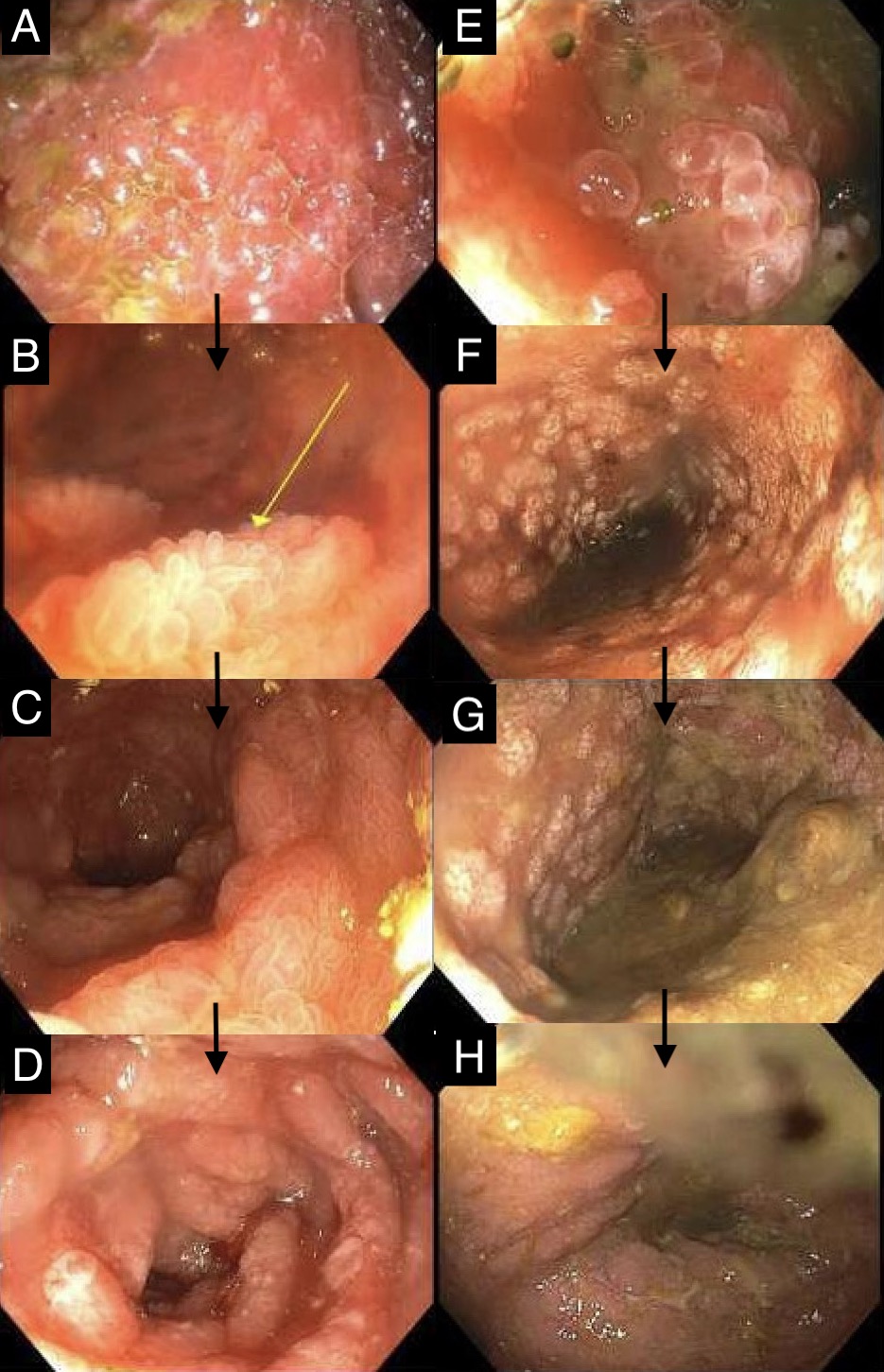
Figure: Figure 1: Progressive improvement of duodenal graft mucosa from denuded, edematous and inflamed mucosa (A) to recovery of epithelial cells and villi (yellow arrow) (B, C) to complete re-epithelization (D); Improvement of ascending colon graft from denuded and ulcerated mucosa (E) to formation of scattered mucosal islands demonstrating early re-epithelialization (F, G) to graft recovery (H).
Disclosures:
Michael Gianarakis indicated no relevant financial relationships.
Robert Carroll indicated no relevant financial relationships.
Michael Gianarakis, MD1, Robert E. Carroll, MD2. P4146 - Vedolizumab for Severe Refractory Acute Cellular Rejection After Intestinal Transplantation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Michael Gianarakis, MD1, Robert E. Carroll, MD2
1University of Illinois at Chicago, Chicago, IL; 2University of Illinois at Chicago, Department of Gastroenterology and Hepatology, Chicago, IL
Introduction: Despite advances in immunotherapy, acute cellular rejection (ACR) affects 30–40% of adult intestinal transplant (ITx) recipients. ACR is traditionally managed with high-dose corticosteroids and intensified immunosuppression. With the development of monoclonal antibodies targeting CD3, CD20, CD52, IL-2, and TNF-alpha, biologics are increasingly used for induction, maintenance, and anti-rejection therapy. Vedolizumab (VDZ), an anti-integrin α4β7 monoclonal antibody approved for inflammatory bowel disease (IBD), has gut-specific immunomodulatory effects that may offer a therapeutic option in intestinal graft rejection. We present a case of severe, treatment-refractory ACR successfully managed with VDZ.
Case Description/
Methods: A 19-year-old male developed short bowel syndrome after massive intestinal resection due to an abdominal gunshot wound and subsequently underwent ITx, including small intestine and ascending colon. He was continued on teduglutide, a glucagon-like peptide 2 analog (GLP-2) post-transplant and presented for diverting loop ileostomy reversal four months post-ITx. Surgical pathology showed mild ACR, for which infliximab and high-dose methylprednisolone were given. He was discharged on a prednisone taper with ongoing tacrolimus and monthly basiliximab. Two days later, he returned with abdominal pain and distension due to small bowel obstruction. He underwent limited small bowel resection with anastomosis and cecostomy creation. Endoscopy revealed denuded, edematous and ulcerated duodenal and colonic graft mucosa, and histology confirmed severe ACR. He received IV VDZ 300 mg, eculizumab 900 mg, and methylprednisolone 500 mg. Within four days of VDZ, serial endoscopies showed marked improvement in mucosal edema and inflammation with re-epithelialization (Figure 1). The graft continues to show endoscopic and histologic recovery following two doses of VDZ for ACR.
Discussion: This case highlights VDZ as a safe and effective treatment option for ACR in ITx. This is the third reported case of VDZ used for ACR in ITx and the first in a non-IBD patient. Our patient responded rapidly to VDZ after failing high-dose methylprednisolone, infliximab and basiliximab. VDZ’s gut-selective mechanism may allow for targeted immunosuppression with fewer systemic effects. Additionally, we postulate that GLP-2 analogs used in the post-transplant period may aid epithelial protection and mucosal recovery during ACR. Further studies are needed to assess long-term efficacy and safety of VDZ in this setting.

Figure: Figure 1: Progressive improvement of duodenal graft mucosa from denuded, edematous and inflamed mucosa (A) to recovery of epithelial cells and villi (yellow arrow) (B, C) to complete re-epithelization (D); Improvement of ascending colon graft from denuded and ulcerated mucosa (E) to formation of scattered mucosal islands demonstrating early re-epithelialization (F, G) to graft recovery (H).
Disclosures:
Michael Gianarakis indicated no relevant financial relationships.
Robert Carroll indicated no relevant financial relationships.
Michael Gianarakis, MD1, Robert E. Carroll, MD2. P4146 - Vedolizumab for Severe Refractory Acute Cellular Rejection After Intestinal Transplantation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

