Monday Poster Session
Category: Small Intestine
P4076 - Evaluation of Larazotide as a Non-Dietary Pharmacological Option for Patients With Celiac Disease: A Meta-Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- MA
Mageda Al Areqi, MD
Raritan Bay Medical Center
Perth Amboy, NJ
Presenting Author(s)
Rana H. Shembesh, MBBCh1, Abdallah Khashan, MD2, Mohammed S. Beshr, MBBS3, Mageda Al Areqi, MD4, Bisher Sawaf, MD5, Muhammed Elhadi, MD6
1Libyan International Medical University, Faculty of Medicine, Benghazi, Benghazi, Libya; 2Capital Health Regional Medical Center, Trenton, NJ; 3Sana’a University, Faculty of Medicine and Health Sciences, Sana'a, Hadramawt, Yemen; 4Raritan Bay Medical Center, Perth Amboy, NJ; 5University of Toledo Medical Center, Toledo, OH; 6College of Medicine, Korea University, Seongbuk, Seoul-t'ukpyolsi, Republic of Korea
Introduction: A gluten-free diet remains the primary treatment for celiac disease, as no pharmacologic therapies have been approved for routine use. Larazotide has shown promise in recent clinical trials and animal studies by regulating tight junctions in intestinal epithelial cells, preserving barrier integrity, and limiting gliadin transport to the lamina propria. This meta-analysis evaluates the efficacy of Larazotide as a non-dietary treatment option for celiac disease.
Methods: On February 15, 2025, we searched PubMed, Scopus, and Cochrane for human clinical trials evaluating Larazotide versus placebo in celiac disease patients. Our outcomes were the lactulose-to-mannitol (LAMA) urinary excretion ratio, which is used to evaluate intestinal permeability. The change in the total Gastrointestinal Symptom Rating Scale (GSRS), a 15-point questionnaire completed by patients to evaluate the severity of gastrointestinal symptoms, and the Celiac Disease-Gastrointestinal Symptom Rating Scale (CD-GSRS), which is similar to the GSRS but tailored to celiac disease. Analysis was performed using RevMan with a random-effects model, and treatment effects were reported as mean differences (MD) with 95% confidence intervals
Results: Out of 99 screened articles, only 4 met the inclusion criteria, encompassing 633 patients. All studies administered a gluten challenge using capsules containing 2.5–2.7 g/day of gluten in both arms. Analysis of the lactulose-to-mannitol (LAMA) urinary excretion ratio revealed no significant difference between Larazotide and placebo (MD: 0.15, 95% CI: -0.56 to 0.25, p = 0.46). Subgroup analysis comparing the 4 mg dose to 8 or 12 mg also showed no significant difference (p = 0.52). Similarly, the Gastrointestinal Symptom Rating Scale (GSRS) (MD: -0.20, CI: -0.50 to 0.09, p = 0.44) and the Celiac Disease-GSRS (MD: -0.13, CI: -0.42 to 0.16, p = 0.41) showed no statistical differences between groups. These findings remained consistent in dose-based subgroup analyses. These results remained non-significant in the subgroup analysis based on dose (4 mg vs. 8 mg or 12 mg).
Discussion: There was no significant difference in the LAMA ratio between the Larazotide and placebo groups. Similar results were observed in our evaluation of the GSRS and CD-GSRS scales. A new, non-dietary novel treatment for celiac disease is still needed.
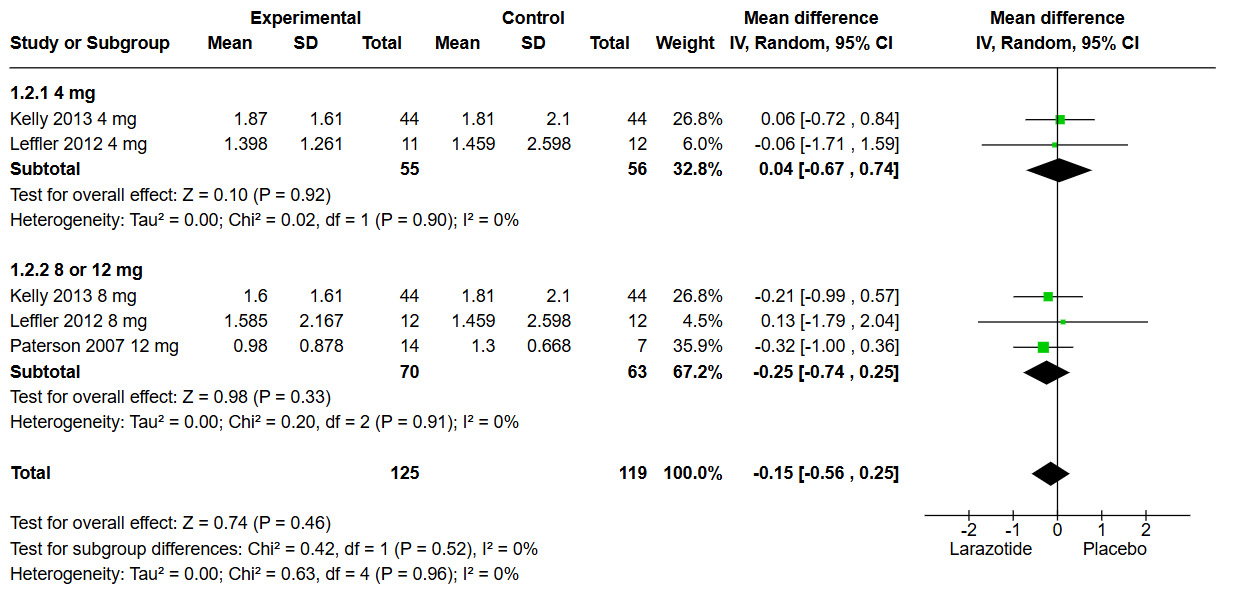
Figure: Figure 1: lactulose-to-mannitol (LAMA) urinary excretion ratio Subgroup Analysis
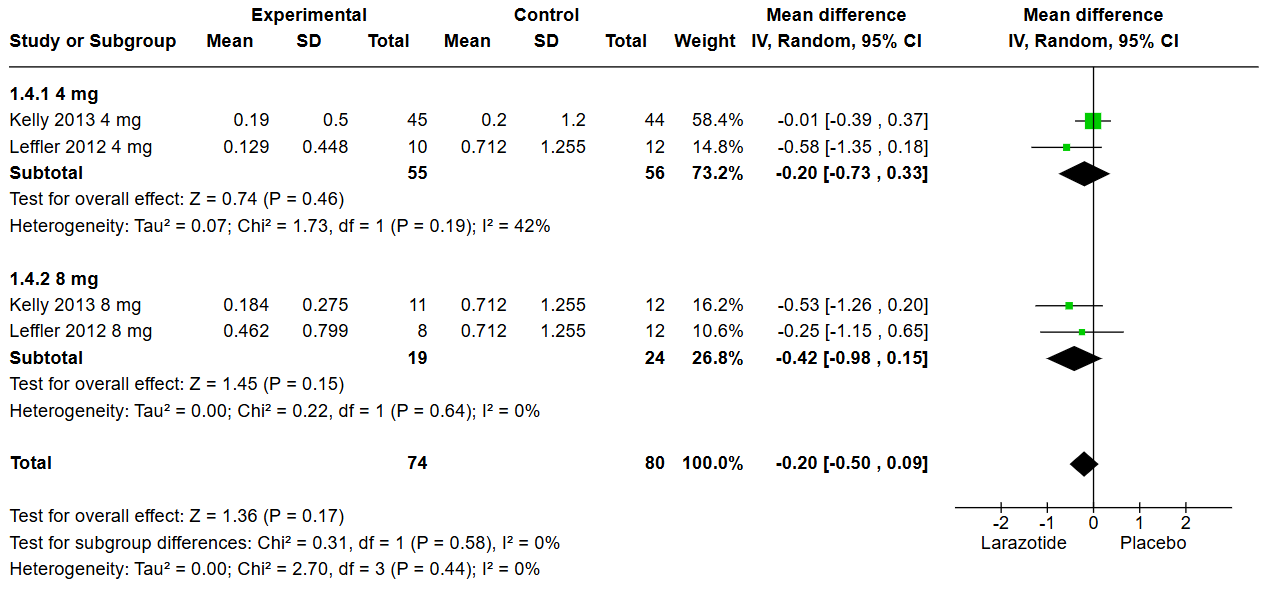
Figure: Figure 2: Gastrointestinal Symptom Rating Scale GSRS Subgroup analysis
Disclosures:
Rana Shembesh indicated no relevant financial relationships.
Abdallah Khashan indicated no relevant financial relationships.
Mohammed Beshr indicated no relevant financial relationships.
Mageda Al Areqi indicated no relevant financial relationships.
Bisher Sawaf indicated no relevant financial relationships.
Muhammed Elhadi indicated no relevant financial relationships.
Rana H. Shembesh, MBBCh1, Abdallah Khashan, MD2, Mohammed S. Beshr, MBBS3, Mageda Al Areqi, MD4, Bisher Sawaf, MD5, Muhammed Elhadi, MD6. P4076 - Evaluation of Larazotide as a Non-Dietary Pharmacological Option for Patients With Celiac Disease: A Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Libyan International Medical University, Faculty of Medicine, Benghazi, Benghazi, Libya; 2Capital Health Regional Medical Center, Trenton, NJ; 3Sana’a University, Faculty of Medicine and Health Sciences, Sana'a, Hadramawt, Yemen; 4Raritan Bay Medical Center, Perth Amboy, NJ; 5University of Toledo Medical Center, Toledo, OH; 6College of Medicine, Korea University, Seongbuk, Seoul-t'ukpyolsi, Republic of Korea
Introduction: A gluten-free diet remains the primary treatment for celiac disease, as no pharmacologic therapies have been approved for routine use. Larazotide has shown promise in recent clinical trials and animal studies by regulating tight junctions in intestinal epithelial cells, preserving barrier integrity, and limiting gliadin transport to the lamina propria. This meta-analysis evaluates the efficacy of Larazotide as a non-dietary treatment option for celiac disease.
Methods: On February 15, 2025, we searched PubMed, Scopus, and Cochrane for human clinical trials evaluating Larazotide versus placebo in celiac disease patients. Our outcomes were the lactulose-to-mannitol (LAMA) urinary excretion ratio, which is used to evaluate intestinal permeability. The change in the total Gastrointestinal Symptom Rating Scale (GSRS), a 15-point questionnaire completed by patients to evaluate the severity of gastrointestinal symptoms, and the Celiac Disease-Gastrointestinal Symptom Rating Scale (CD-GSRS), which is similar to the GSRS but tailored to celiac disease. Analysis was performed using RevMan with a random-effects model, and treatment effects were reported as mean differences (MD) with 95% confidence intervals
Results: Out of 99 screened articles, only 4 met the inclusion criteria, encompassing 633 patients. All studies administered a gluten challenge using capsules containing 2.5–2.7 g/day of gluten in both arms. Analysis of the lactulose-to-mannitol (LAMA) urinary excretion ratio revealed no significant difference between Larazotide and placebo (MD: 0.15, 95% CI: -0.56 to 0.25, p = 0.46). Subgroup analysis comparing the 4 mg dose to 8 or 12 mg also showed no significant difference (p = 0.52). Similarly, the Gastrointestinal Symptom Rating Scale (GSRS) (MD: -0.20, CI: -0.50 to 0.09, p = 0.44) and the Celiac Disease-GSRS (MD: -0.13, CI: -0.42 to 0.16, p = 0.41) showed no statistical differences between groups. These findings remained consistent in dose-based subgroup analyses. These results remained non-significant in the subgroup analysis based on dose (4 mg vs. 8 mg or 12 mg).
Discussion: There was no significant difference in the LAMA ratio between the Larazotide and placebo groups. Similar results were observed in our evaluation of the GSRS and CD-GSRS scales. A new, non-dietary novel treatment for celiac disease is still needed.

Figure: Figure 1: lactulose-to-mannitol (LAMA) urinary excretion ratio Subgroup Analysis

Figure: Figure 2: Gastrointestinal Symptom Rating Scale GSRS Subgroup analysis
Disclosures:
Rana Shembesh indicated no relevant financial relationships.
Abdallah Khashan indicated no relevant financial relationships.
Mohammed Beshr indicated no relevant financial relationships.
Mageda Al Areqi indicated no relevant financial relationships.
Bisher Sawaf indicated no relevant financial relationships.
Muhammed Elhadi indicated no relevant financial relationships.
Rana H. Shembesh, MBBCh1, Abdallah Khashan, MD2, Mohammed S. Beshr, MBBS3, Mageda Al Areqi, MD4, Bisher Sawaf, MD5, Muhammed Elhadi, MD6. P4076 - Evaluation of Larazotide as a Non-Dietary Pharmacological Option for Patients With Celiac Disease: A Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
