Monday Poster Session
Category: Liver
P3996 - Hormonal Therapy and Hepatic Risk: A Case Report of Progestin-Induced Cholestatic Liver Injury
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Megan Carugati, DO (she/her/hers)
University of Connecticut Health Center
Farmington, CT
Presenting Author(s)
Megan Carugati, DO1, Geraldine Nabeta, MD1, Anjana Abraham, DO1, Madura Saravanan, MD2
1University of Connecticut Health Center, Farmington, CT; 2Hospital of Central Connecticut, New Britain, CT
Introduction: Medroxyprogesterone acetone is a synthetic version of the naturally occurring hormone progesterone widely used for various gynecologic conditions. It is commonly indicated for contraception, menstrual cycle disorders, hormone replacement, cancer risk reduction, acute uterine bleeding, and endometrial hyperplasia. At increased doses, synthetic progesterones (progestins) alone have been shown to cause a hepatocellular and rarely cholestatic pattern of liver injury, one to two weeks after administration. The mechanism of how progestin therapy induces liver injury is poorly understood. Here we present a case of a postmenopausal woman with end-stage liver disease and abnormal uterine bleeding requiring progestin therapy, who subsequently sustained an unusual pattern of liver injury.
Case Description/
Methods: The patient is a 64-year-old female with known HIV on Biktarvy, CKD, MASH cirrhosis decompensated by ascites, non-bleeding esophageal varices, and hepatic encephalopathy with prior listing for dual kidney/liver transplant, who was admitted for postmenopausal bleeding in the setting of new cervical/uterine mass. Biopsies of the lesions reported CIN and vulvar HSIL/VIN 3. Following a subsequent endometrial curettage and partial vulvectomy, she had no further evidence of malignancy. Her postoperative course was complicated by profound vaginal bleeding requiring several units of blood transfusions, likely due to coagulopathy from cirrhosis. She was started on Provera with good effect in controlling her bleeding, however her liver enzymes, bilirubin in particular, acutely worsened; AST/ALT 91/31, ALP 143 total bilirubin 19. MRCP was negative for dilated bile ducts. The patient’s Roussell Uclaf Causality Assessment Method score was 9, indicating a probable case of progestin-induced liver injury. Provera was discontinued in favor of a hormonal intrauterine device with noted improvement in direct hyperbilirubinemia. With a MELD 3.0 of 37, she was reactivated on the transplant list and successfully underwent a dual kidney/liver transplant.
Discussion: Although hormonal hepatotoxicity is well studied, progestin-induced cholestatic liver injury in particular is largely rare. Clinicians should maintain a high level of suspicion when evaluating patients on hormonal therapy with acutely worsening liver enzymes, regardless of the pattern of injury. It is critical to consider various differentials, in order to optimize treatment in medically complex presentations.
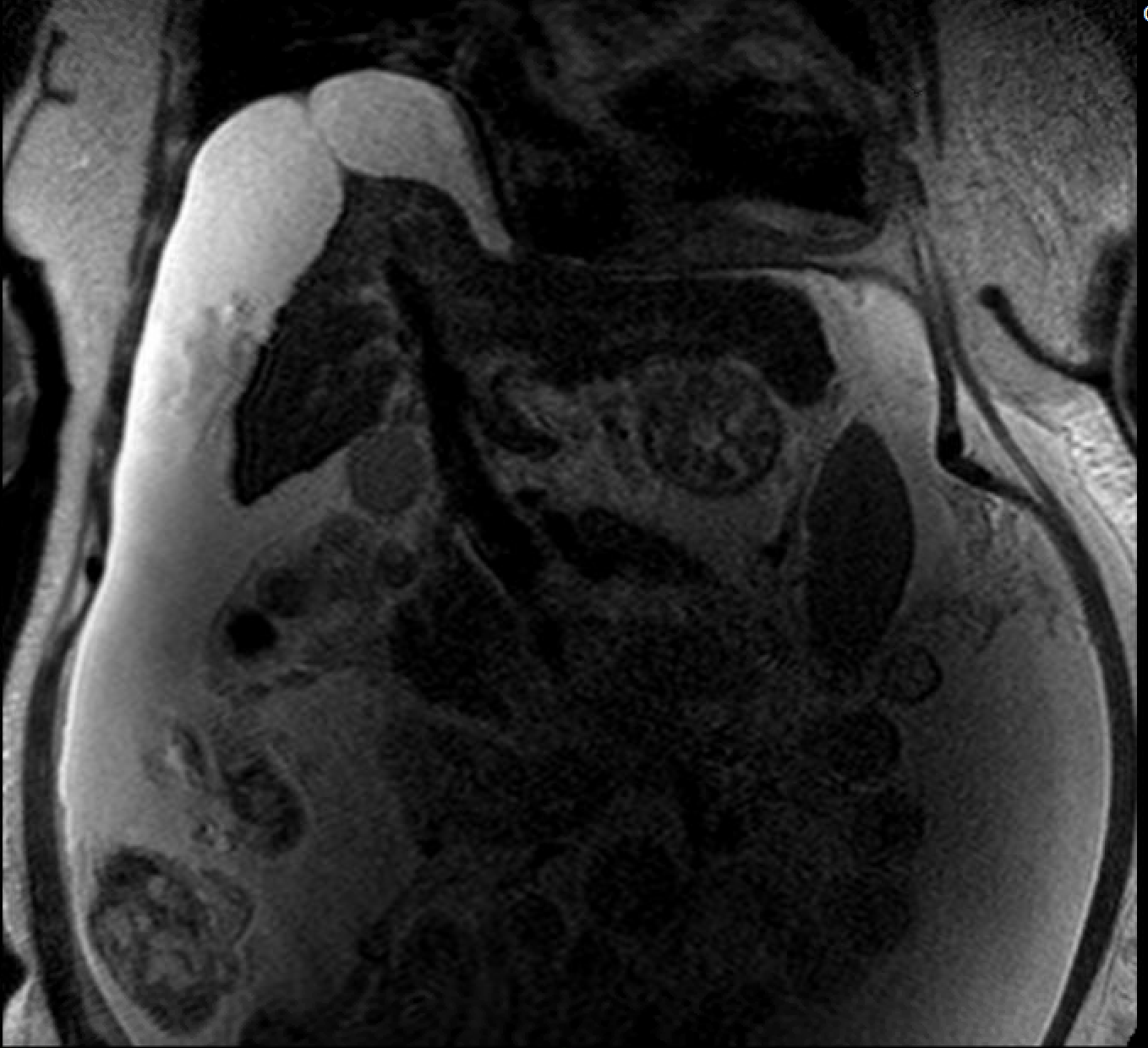
Figure: Figure 1: T2 weighted MRI abdomen with patent biliary tree and no dilated ducts.
Disclosures:
Megan Carugati indicated no relevant financial relationships.
Geraldine Nabeta indicated no relevant financial relationships.
Anjana Abraham indicated no relevant financial relationships.
Madura Saravanan indicated no relevant financial relationships.
Megan Carugati, DO1, Geraldine Nabeta, MD1, Anjana Abraham, DO1, Madura Saravanan, MD2. P3996 - Hormonal Therapy and Hepatic Risk: A Case Report of Progestin-Induced Cholestatic Liver Injury, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Connecticut Health Center, Farmington, CT; 2Hospital of Central Connecticut, New Britain, CT
Introduction: Medroxyprogesterone acetone is a synthetic version of the naturally occurring hormone progesterone widely used for various gynecologic conditions. It is commonly indicated for contraception, menstrual cycle disorders, hormone replacement, cancer risk reduction, acute uterine bleeding, and endometrial hyperplasia. At increased doses, synthetic progesterones (progestins) alone have been shown to cause a hepatocellular and rarely cholestatic pattern of liver injury, one to two weeks after administration. The mechanism of how progestin therapy induces liver injury is poorly understood. Here we present a case of a postmenopausal woman with end-stage liver disease and abnormal uterine bleeding requiring progestin therapy, who subsequently sustained an unusual pattern of liver injury.
Case Description/
Methods: The patient is a 64-year-old female with known HIV on Biktarvy, CKD, MASH cirrhosis decompensated by ascites, non-bleeding esophageal varices, and hepatic encephalopathy with prior listing for dual kidney/liver transplant, who was admitted for postmenopausal bleeding in the setting of new cervical/uterine mass. Biopsies of the lesions reported CIN and vulvar HSIL/VIN 3. Following a subsequent endometrial curettage and partial vulvectomy, she had no further evidence of malignancy. Her postoperative course was complicated by profound vaginal bleeding requiring several units of blood transfusions, likely due to coagulopathy from cirrhosis. She was started on Provera with good effect in controlling her bleeding, however her liver enzymes, bilirubin in particular, acutely worsened; AST/ALT 91/31, ALP 143 total bilirubin 19. MRCP was negative for dilated bile ducts. The patient’s Roussell Uclaf Causality Assessment Method score was 9, indicating a probable case of progestin-induced liver injury. Provera was discontinued in favor of a hormonal intrauterine device with noted improvement in direct hyperbilirubinemia. With a MELD 3.0 of 37, she was reactivated on the transplant list and successfully underwent a dual kidney/liver transplant.
Discussion: Although hormonal hepatotoxicity is well studied, progestin-induced cholestatic liver injury in particular is largely rare. Clinicians should maintain a high level of suspicion when evaluating patients on hormonal therapy with acutely worsening liver enzymes, regardless of the pattern of injury. It is critical to consider various differentials, in order to optimize treatment in medically complex presentations.

Figure: Figure 1: T2 weighted MRI abdomen with patent biliary tree and no dilated ducts.
Disclosures:
Megan Carugati indicated no relevant financial relationships.
Geraldine Nabeta indicated no relevant financial relationships.
Anjana Abraham indicated no relevant financial relationships.
Madura Saravanan indicated no relevant financial relationships.
Megan Carugati, DO1, Geraldine Nabeta, MD1, Anjana Abraham, DO1, Madura Saravanan, MD2. P3996 - Hormonal Therapy and Hepatic Risk: A Case Report of Progestin-Induced Cholestatic Liver Injury, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

