Monday Poster Session
Category: Liver
P3981 - A Novel Case of Cholestatic Drug-Induced Liver Injury Following Filgrastim Use in a Patient With Neutropenic Fever
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Mohammed S. Zaman, DO
Mercyhealth Rockford
Rockford, IL
Presenting Author(s)
Mohammed Zaman, DO1, Sultan Ahmed, DO1, Saad Rashid, MD1, Ahmet Sakiri, MD1, Luqman Baloch, MD2, Altaf Dawood, MD3
1Mercyhealth Rockford, Rockford, IL; 2Mercyhealth, Rockford, IL; 3Mercyhealth Gastroenterology, Rockford, IL
Introduction: Drug-induced liver injury (DILI) is a challenging diagnosis due to its variable presentation and extensive work-up. Filgrastim - often used for chemotherapy-induced neutropenia – is generally well tolerated with varying side effects; however, significant hepatotoxicity is rare. This report describes a unique case of DILI following filgrastim use in the inpatient setting.
Case Description/
Methods: A 70-year-old male with Waldenström’s macroglobulinemia, undergoing monthly chemotherapy with bendamustine and rituximab followed by D1 C2 Ruxience, presented three weeks after his fifth infusion, with neutropenic fever. He was febrile to 101°F, HR 140 and BP 95/52. He received 30 cc/kg of Lactated Ringer's, IV vancomycin, and cefepime. Additional antibiotics included meropenem, micafungin, and piperacillin-tazobactam. Infectious workup was unremarkable, and antibiotic regimens were stopped. Filgrastim was given on day two of admission for six days. Initial hepatic panel showed ALT 13 U/L, AST 15 U/L, ALP 155 U/L, and total bilirubin (TB) 0.6 mg/dL. After one week of filgrastim initiation, ALP was 1000 U/L, ALT 187 U/L, AST 264 U/L, and TB 5.5 mg/dL. Viral and autoimmune serologies, abdominal ultrasound, and MRCP were negative. Three days after stopping filgrastim, labs trended down (Figure 1). Liver biopsy was therefore deferred. Per the Roussell Uclaf Causality Assessment Method (RUCAM) scale, filgrastim was deemed the probable cause for DILI. He was later discharged in stable condition.
Discussion: This case underscores filgrastim as a rare but potential cause of DILI. Prior reports are uncommon, with only one reported case, supported by a RUCAM score and similar transaminase pattern [1]. Although he received antibiotics, labs remained stable for the first 72 hours before rapidly climbing. Furthermore, he had received those antibiotics in prior stays, without evidence of DILI, and the elevation continued after antibiotic cessation. Chemotherapy was considered a cause; however, he had received the agents prior to admission without noted hepatotoxicity. The patient’s improvement upon filgrastim withdrawal supports its incrimination. Clinicians should recognize DILI in the differential diagnosis of transaminase elevation, even when commonly implicated drugs are not administered.
1. Sharma D, Da BL, Vittal A, Kapuria D, Heller T, Ben Yakov G. Stimulating More Than Just the Granulocytes: Drug-Induced Liver Injury From Filgrastim. ACG Case Rep J. 2019;6(6):e00098. doi:10.14309/crj.0000000000000098.
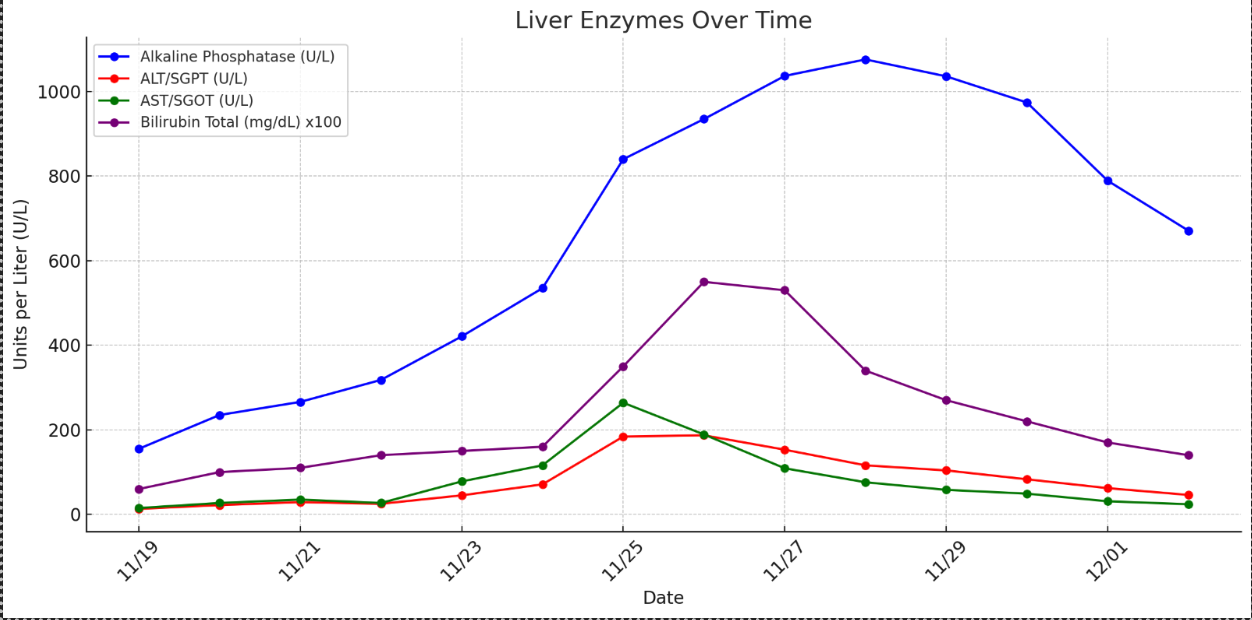
Figure: Trends of Liver Enzymes and Total Bilirubin
Disclosures:
Mohammed Zaman indicated no relevant financial relationships.
Sultan Ahmed indicated no relevant financial relationships.
Saad Rashid indicated no relevant financial relationships.
Ahmet Sakiri indicated no relevant financial relationships.
Luqman Baloch indicated no relevant financial relationships.
Altaf Dawood indicated no relevant financial relationships.
Mohammed Zaman, DO1, Sultan Ahmed, DO1, Saad Rashid, MD1, Ahmet Sakiri, MD1, Luqman Baloch, MD2, Altaf Dawood, MD3. P3981 - A Novel Case of Cholestatic Drug-Induced Liver Injury Following Filgrastim Use in a Patient With Neutropenic Fever, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mercyhealth Rockford, Rockford, IL; 2Mercyhealth, Rockford, IL; 3Mercyhealth Gastroenterology, Rockford, IL
Introduction: Drug-induced liver injury (DILI) is a challenging diagnosis due to its variable presentation and extensive work-up. Filgrastim - often used for chemotherapy-induced neutropenia – is generally well tolerated with varying side effects; however, significant hepatotoxicity is rare. This report describes a unique case of DILI following filgrastim use in the inpatient setting.
Case Description/
Methods: A 70-year-old male with Waldenström’s macroglobulinemia, undergoing monthly chemotherapy with bendamustine and rituximab followed by D1 C2 Ruxience, presented three weeks after his fifth infusion, with neutropenic fever. He was febrile to 101°F, HR 140 and BP 95/52. He received 30 cc/kg of Lactated Ringer's, IV vancomycin, and cefepime. Additional antibiotics included meropenem, micafungin, and piperacillin-tazobactam. Infectious workup was unremarkable, and antibiotic regimens were stopped. Filgrastim was given on day two of admission for six days. Initial hepatic panel showed ALT 13 U/L, AST 15 U/L, ALP 155 U/L, and total bilirubin (TB) 0.6 mg/dL. After one week of filgrastim initiation, ALP was 1000 U/L, ALT 187 U/L, AST 264 U/L, and TB 5.5 mg/dL. Viral and autoimmune serologies, abdominal ultrasound, and MRCP were negative. Three days after stopping filgrastim, labs trended down (Figure 1). Liver biopsy was therefore deferred. Per the Roussell Uclaf Causality Assessment Method (RUCAM) scale, filgrastim was deemed the probable cause for DILI. He was later discharged in stable condition.
Discussion: This case underscores filgrastim as a rare but potential cause of DILI. Prior reports are uncommon, with only one reported case, supported by a RUCAM score and similar transaminase pattern [1]. Although he received antibiotics, labs remained stable for the first 72 hours before rapidly climbing. Furthermore, he had received those antibiotics in prior stays, without evidence of DILI, and the elevation continued after antibiotic cessation. Chemotherapy was considered a cause; however, he had received the agents prior to admission without noted hepatotoxicity. The patient’s improvement upon filgrastim withdrawal supports its incrimination. Clinicians should recognize DILI in the differential diagnosis of transaminase elevation, even when commonly implicated drugs are not administered.
1. Sharma D, Da BL, Vittal A, Kapuria D, Heller T, Ben Yakov G. Stimulating More Than Just the Granulocytes: Drug-Induced Liver Injury From Filgrastim. ACG Case Rep J. 2019;6(6):e00098. doi:10.14309/crj.0000000000000098.

Figure: Trends of Liver Enzymes and Total Bilirubin
Disclosures:
Mohammed Zaman indicated no relevant financial relationships.
Sultan Ahmed indicated no relevant financial relationships.
Saad Rashid indicated no relevant financial relationships.
Ahmet Sakiri indicated no relevant financial relationships.
Luqman Baloch indicated no relevant financial relationships.
Altaf Dawood indicated no relevant financial relationships.
Mohammed Zaman, DO1, Sultan Ahmed, DO1, Saad Rashid, MD1, Ahmet Sakiri, MD1, Luqman Baloch, MD2, Altaf Dawood, MD3. P3981 - A Novel Case of Cholestatic Drug-Induced Liver Injury Following Filgrastim Use in a Patient With Neutropenic Fever, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
