Monday Poster Session
Category: Liver
P3934 - Hepatotoxicity Linked to Compounded Semaglutide: A Rare Case of Probable Drug-Induced Liver Injury
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Nikita Garg, MD (she/her/hers)
Geisinger Wyoming Valley Medical Center
Wilkes-Barre, PA
Presenting Author(s)
Nikita Garg, MD, John Samies, DO, Duane Deivert, DO, FACG
Geisinger Wyoming Valley Medical Center, Wilkes-Barre, PA
Introduction: Semaglutide, a glucagon-like peptide-1 (GLP-1) has been shown to improve liver enzymes with metabolic dysfunction associated steatotic liver disease (MASLD) and metabolic dysfunction associated steatohepatitis (MASH). Semaglutide-induced liver injury is rarely reported. We present a rare case of probable drug induced liver injury (DILI) associated with compounded semaglutide.
Case Description/
Methods: A 58-year-old male was referred to hepatology clinic for incidentally detected elevation in liver chemistries. Due to insurance limitations on coverage of weight-loss medications, the patient had procured compounded semaglutide from a direct-to-consumer telehealth platform. He administered increasing weekly doses (10 mg, 20 mg, 30 mg, and 40 mg) over a four-week period. Approximately one week after the last dose, routine laboratory workup revealed significant elevations in liver enzymes consistent with hepatocellular injury: AST 1184 U/L, ALT 944 U/L, ALP 261 U/L, and total bilirubin 2.3 mg/dl. The patient discontinued the medication thereafter. He was asymptomatic throughout and there was no concurrent illness, shock, or heart failure. He denied alcohol use, herbal or over-the-counter supplement intake. Extensive workup excluded viral, autoimmune, and obstructive etiologies. Following drug cessation, liver enzymes progressively normalized over the next two months.
Discussion: Semaglutide was deemed the probable cause of DILI in our patient, supported by a Roussel Uclaf Causality Assessment Method score of 8, temporal correlation with drug initiation, resolution upon withdrawal, and absence of alternative etiologies. The exact cause of semaglutide-induced liver injury remains unclear. While semaglutide has been linked to liver stress from rapid weight loss and biliary disease in previous case reports, our patient lacked these risk factors. Notably, our patient had been using compounded semaglutide—an unregulated formulation often accessible without a prescription through online platforms, prepared with semaglutide base or acetate instead of the Food and Drug Administration-approved sodium salt, and potentially containing variable excipients that can affect bioavailability, metabolism, and toxicity. This case underscores importance of clinician vigilance and routine liver function monitoring in patients using compounded semaglutide as this poses a potential health threat to patients.
Sanyal AJ et al. Phase 3 Trial of Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis. N Engl J Med 2025.
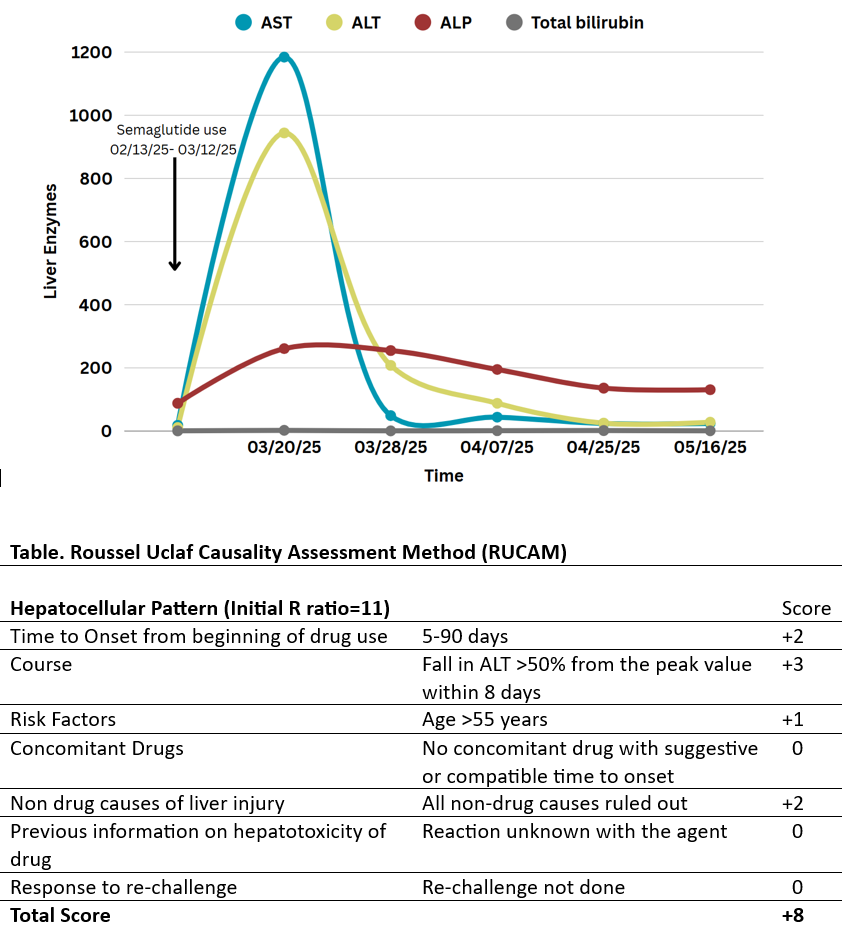
Figure: Figure.1 Temporal relationship between Semaglutide use and liver enzyme elevations with corresponding RUCAM-based causality assessment. [AST= Aspartate transaminase, ALT=Alanine transaminase, ALP= Alkaline Phosphatase]
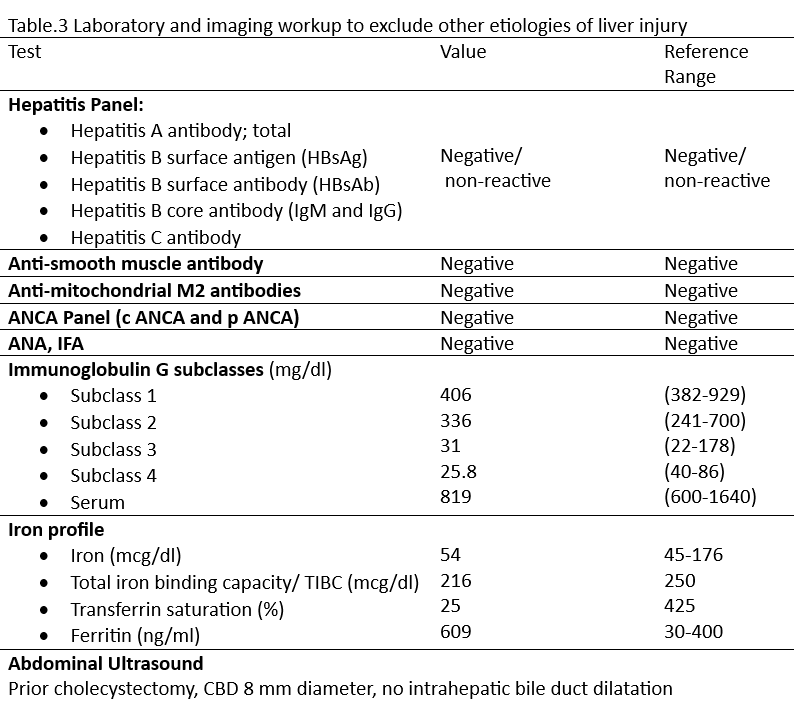
Figure: Figure.2 Comprehensive laboratory evaluation to exclude alternative etiologies of liver injury. [ANCA= Antineutrophil cytoplasmic antibodies; ANA= Antinuclear antibodies; IFA= Immunofluorescence Assay; CBD= Common bile duct]
Disclosures:
Nikita Garg indicated no relevant financial relationships.
John Samies indicated no relevant financial relationships.
Duane Deivert: Amgen Inc – Stock-publicly held company(excluding mutual/index funds). BioLife Sciences – Stock-publicly held company(excluding mutual/index funds). BioNTech – Stock-publicly held company(excluding mutual/index funds). Eli Lilly – Stock-publicly held company(excluding mutual/index funds). Exact Sciences – Stock-publicly held company(excluding mutual/index funds). Finch Therapeutics – Stock-publicly held company(excluding mutual/index funds). Gilead Sciences – Stock-publicly held company(excluding mutual/index funds). Guardant Health – Stock-publicly held company(excluding mutual/index funds). Madrigal Pharmaceuticals – Stock-publicly held company(excluding mutual/index funds). Medtronic – Stock-publicly held company(excluding mutual/index funds). Merck & Co – Stock-publicly held company(excluding mutual/index funds). Moderna – Stock-publicly held company(excluding mutual/index funds). Novo Nordisk – Stock-publicly held company(excluding mutual/index funds). Pfizer – Stock-publicly held company(excluding mutual/index funds). Regeneron – Stock-publicly held company(excluding mutual/index funds). Seres Therapeutics – Stock-publicly held company(excluding mutual/index funds). Structure Therapeutics – Stock-publicly held company(excluding mutual/index funds). Teladoc Health – Stock-publicly held company(excluding mutual/index funds). Thermo Fisher Scientific – Stock-publicly held company(excluding mutual/index funds). Viking Therapeutics – Stock-publicly held company(excluding mutual/index funds).
Nikita Garg, MD, John Samies, DO, Duane Deivert, DO, FACG. P3934 - Hepatotoxicity Linked to Compounded Semaglutide: A Rare Case of Probable Drug-Induced Liver Injury, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Geisinger Wyoming Valley Medical Center, Wilkes-Barre, PA
Introduction: Semaglutide, a glucagon-like peptide-1 (GLP-1) has been shown to improve liver enzymes with metabolic dysfunction associated steatotic liver disease (MASLD) and metabolic dysfunction associated steatohepatitis (MASH). Semaglutide-induced liver injury is rarely reported. We present a rare case of probable drug induced liver injury (DILI) associated with compounded semaglutide.
Case Description/
Methods: A 58-year-old male was referred to hepatology clinic for incidentally detected elevation in liver chemistries. Due to insurance limitations on coverage of weight-loss medications, the patient had procured compounded semaglutide from a direct-to-consumer telehealth platform. He administered increasing weekly doses (10 mg, 20 mg, 30 mg, and 40 mg) over a four-week period. Approximately one week after the last dose, routine laboratory workup revealed significant elevations in liver enzymes consistent with hepatocellular injury: AST 1184 U/L, ALT 944 U/L, ALP 261 U/L, and total bilirubin 2.3 mg/dl. The patient discontinued the medication thereafter. He was asymptomatic throughout and there was no concurrent illness, shock, or heart failure. He denied alcohol use, herbal or over-the-counter supplement intake. Extensive workup excluded viral, autoimmune, and obstructive etiologies. Following drug cessation, liver enzymes progressively normalized over the next two months.
Discussion: Semaglutide was deemed the probable cause of DILI in our patient, supported by a Roussel Uclaf Causality Assessment Method score of 8, temporal correlation with drug initiation, resolution upon withdrawal, and absence of alternative etiologies. The exact cause of semaglutide-induced liver injury remains unclear. While semaglutide has been linked to liver stress from rapid weight loss and biliary disease in previous case reports, our patient lacked these risk factors. Notably, our patient had been using compounded semaglutide—an unregulated formulation often accessible without a prescription through online platforms, prepared with semaglutide base or acetate instead of the Food and Drug Administration-approved sodium salt, and potentially containing variable excipients that can affect bioavailability, metabolism, and toxicity. This case underscores importance of clinician vigilance and routine liver function monitoring in patients using compounded semaglutide as this poses a potential health threat to patients.
Sanyal AJ et al. Phase 3 Trial of Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis. N Engl J Med 2025.

Figure: Figure.1 Temporal relationship between Semaglutide use and liver enzyme elevations with corresponding RUCAM-based causality assessment. [AST= Aspartate transaminase, ALT=Alanine transaminase, ALP= Alkaline Phosphatase]

Figure: Figure.2 Comprehensive laboratory evaluation to exclude alternative etiologies of liver injury. [ANCA= Antineutrophil cytoplasmic antibodies; ANA= Antinuclear antibodies; IFA= Immunofluorescence Assay; CBD= Common bile duct]
Disclosures:
Nikita Garg indicated no relevant financial relationships.
John Samies indicated no relevant financial relationships.
Duane Deivert: Amgen Inc – Stock-publicly held company(excluding mutual/index funds). BioLife Sciences – Stock-publicly held company(excluding mutual/index funds). BioNTech – Stock-publicly held company(excluding mutual/index funds). Eli Lilly – Stock-publicly held company(excluding mutual/index funds). Exact Sciences – Stock-publicly held company(excluding mutual/index funds). Finch Therapeutics – Stock-publicly held company(excluding mutual/index funds). Gilead Sciences – Stock-publicly held company(excluding mutual/index funds). Guardant Health – Stock-publicly held company(excluding mutual/index funds). Madrigal Pharmaceuticals – Stock-publicly held company(excluding mutual/index funds). Medtronic – Stock-publicly held company(excluding mutual/index funds). Merck & Co – Stock-publicly held company(excluding mutual/index funds). Moderna – Stock-publicly held company(excluding mutual/index funds). Novo Nordisk – Stock-publicly held company(excluding mutual/index funds). Pfizer – Stock-publicly held company(excluding mutual/index funds). Regeneron – Stock-publicly held company(excluding mutual/index funds). Seres Therapeutics – Stock-publicly held company(excluding mutual/index funds). Structure Therapeutics – Stock-publicly held company(excluding mutual/index funds). Teladoc Health – Stock-publicly held company(excluding mutual/index funds). Thermo Fisher Scientific – Stock-publicly held company(excluding mutual/index funds). Viking Therapeutics – Stock-publicly held company(excluding mutual/index funds).
Nikita Garg, MD, John Samies, DO, Duane Deivert, DO, FACG. P3934 - Hepatotoxicity Linked to Compounded Semaglutide: A Rare Case of Probable Drug-Induced Liver Injury, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
