Monday Poster Session
Category: Liver
P3905 - An Old Problem With a Re-purposed Solution: Novel Application of Emtricitabine-Tenofovir to Treat Persistent HBV Viremia
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Peter D. Block, MD, MSc
NYU Langone Health
New York, NY
Presenting Author(s)
Peter D. Block, MD, MSc, Ceena Chandrabos, MD, Viviana Figueroa-Diaz, MD, Linda Law, MD
NYU Langone Health, New York, NY
Introduction: As multi-level efforts seek to expand indications for – and access to – nucleos(t)ide analogues (NUC) for chronic hepatitis B (CHB), a corresponding uptick in cases of persistent viremia is expected. Persistent hepatitis B virus (HBV) viremia occurs in nearly 20% of cases and is defined as detectable HBV DNA despite 48 weeks of uninterrupted therapy. Its significance is underlined by two interrelated observations: HBV DNA correlates with future risk of liver-related complications, whereas viral suppression with therapy decreases this risk. A defined clinical approach to persistent HBV viremia is needed, as a paucity of literature on this topic limits current guidance.
Described here are four cases of persistent HBV viremia, who were effectively treated with fixed dose combination medications: emtricitabine-tenofovir disoproxil fumarate (FTC-TDF) or emtricitabine-tenofovir alafenamide (FTC-TAF).
Case Description/
Methods: Included cases have CHB without HIV and varying degrees of underlying liver fibrosis (Table 1-A). Two cases have cirrhosis including one with HCC, which all predated FTC-TDF or FTC-TAF therapy. HBV viremia persisted despite a minimum 1-year course of NUC monotherapy across cases, though baseline HBV DNA levels differed them. Different NUC combinations and sequences were universally unsuccessful (Table 1-B). Genetic profiles were obtained, showing variable resistance patterns.
Response to antiviral therapies is depicted (Figure 1). FTC-TDF was prescribed in three cases, while FTC-TAF was provided in the remaining case. Both led to complete viral suppression within 48 weeks of therapy. Non-invasive tests show stability or regression of underlying fibrosis following therapy. Neither de novo HCC nor virological breakthroughs have been seen after long-term follow-up (range of 1-11 years). Similarly, medication-related adverse events have not occurred with either therapy.
Discussion: Persistent HBV viremia is an understudied problem without a defined treatment pathway. As first reported here, FTC-TDF or FTC-TAF can effectively gain virological control in these cases. FTC is a NUC commonly incorporated into HIV prophylactic and treatment regimens, though it also shows antiviral activity against HBV. It is commercially available and readily prescribed in fixed-dose combination tablets. Thus, FTC-TDF and FTC-TAF are attractive options for persistent HBV viremia. Larger scale studies are warranted to validate these findings and inform future treatment algorithms for persistent HBV viremia.
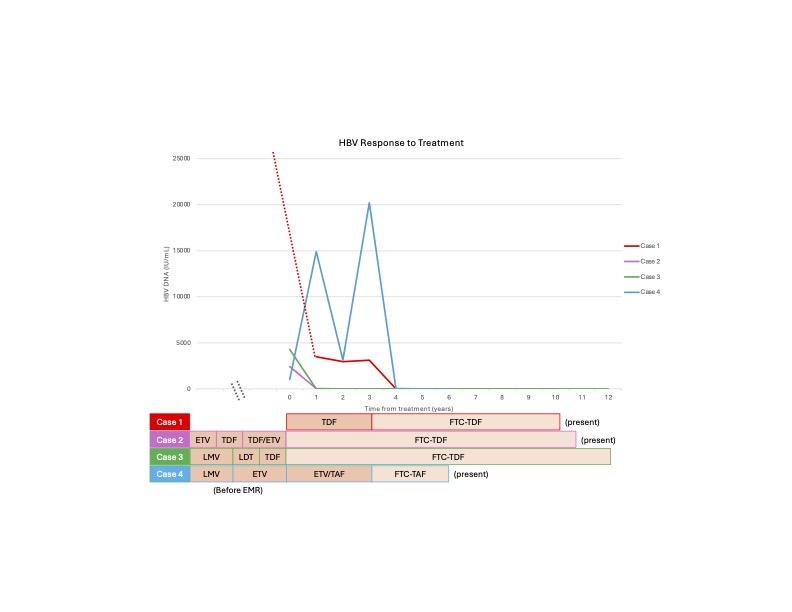
Figure: Table 1A-B. Patient characteristics. Table 1-A summarizes pertinent medical and viral features of each case. This includes clinical information that predated initiation of either FTC-TDF or FTC-TDF. Table 1-B details each case's antiviral treatment regimens. Abbreviations: BCP mutations (Basal Core Mutations); ETV (Entecavir); F3-F4 (Fibrosis stage 3-4; FIB-4 (Fibrosis-4 Score); FTC (Emtricitabine); HBeAb (HBV e Antibody); HBeAg (HBV e Antigen); HBV (Hepatitis B Virus); HCC (Hepatocellular Carcinoma); HIV (Human Immunodeficiency Virus); HCV (Hepatitis C Virus); HDV (Hepatitis Delta Virus); LSM (Liver Sitffness Measurement); N/A (not applicable); TAF (Tenofovir Alafenamide); TDF (Tenofovir Disoproxil Fumarate).
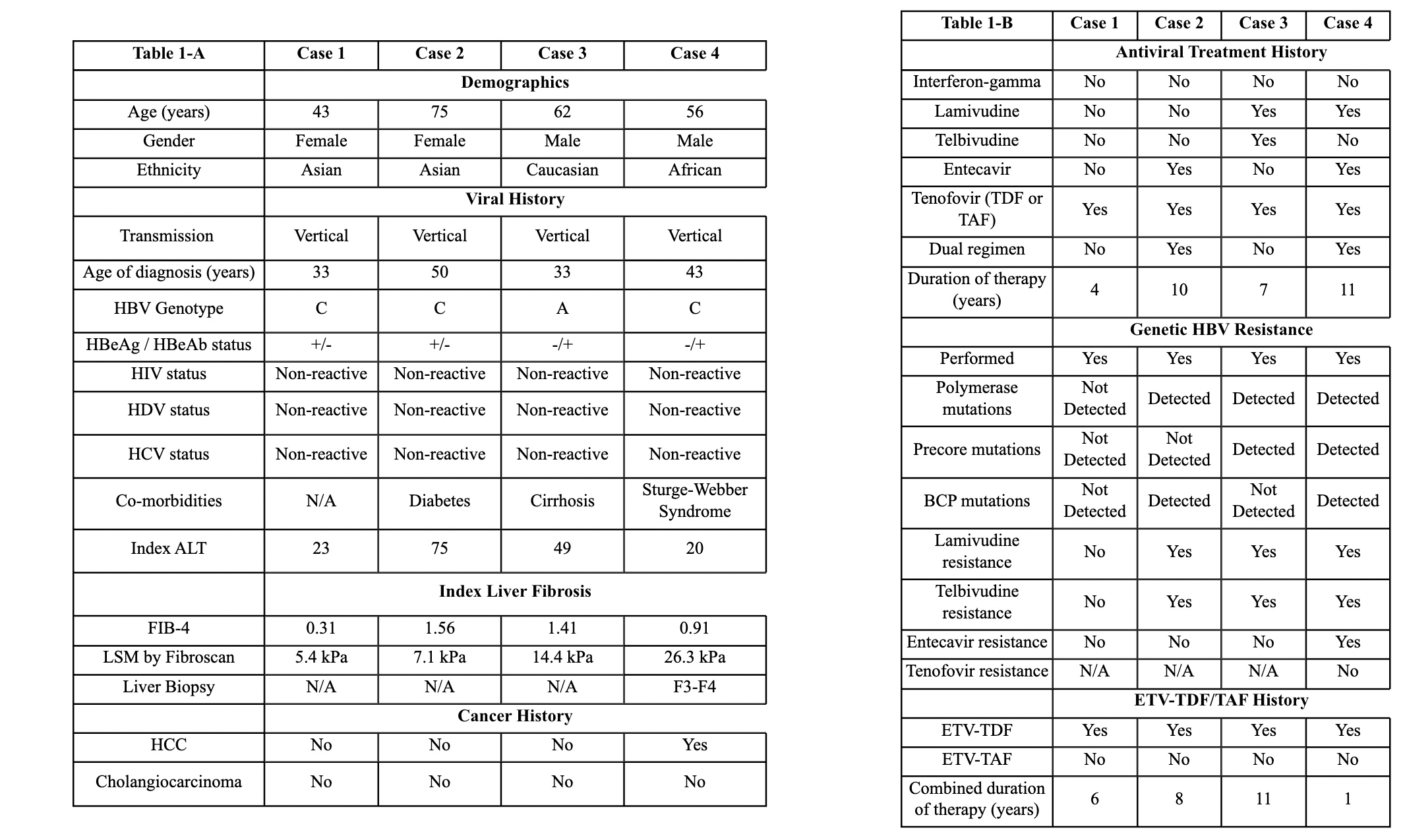
Figure: Figure 1. Viral Response to Treatment over Time. The virological kinetics for each case is graphically depicted with an accompanying treatment table. The cases are color coordinated: Case 1 (red), Case 2 (purple), Case 3 (green), and Case 4 (blue). On the graph, year zero (0) represents the first recorded HBV DNA level in the EMR for each case. The preceding antiviral therapies received before this time point (year 0) are listed in the table without a corresponding HBV DNA level. For case 1, the patient's index HBV DNA level was significantly higher than the other cases (170,000,000 IU/mL) and thus is illustrated by a dotted red line. Note that while all cases have received FTC-TDF or FTC-TAF for different durations, they are all still presently prescribed these medications as indicated in the table. Importantly, HBV DNA levels were consistently elevated with other NUC therapies across all cases, then became undetectable shortly after initiation of either FTC-TDF or FTC-TAF. Abbreviations: EMR (electronic medical records); ETV (Entecavir); FTC-TAF (Emtricitabine-Tenofovir Alafenamide); FTC-TDF (Emtricitabine-Tenofovir Disoproxil Fumarate); IU/mL (international units per milliliter); LDT (Telbivudine); LMV (Lamivudine); NUC (Nucleos(t)ide Analogues).
Disclosures:
Peter Block indicated no relevant financial relationships.
Ceena Chandrabos indicated no relevant financial relationships.
Viviana Figueroa-Diaz indicated no relevant financial relationships.
Linda Law indicated no relevant financial relationships.
Peter D. Block, MD, MSc, Ceena Chandrabos, MD, Viviana Figueroa-Diaz, MD, Linda Law, MD. P3905 - An Old Problem With a Re-purposed Solution: Novel Application of Emtricitabine-Tenofovir to Treat Persistent HBV Viremia, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
NYU Langone Health, New York, NY
Introduction: As multi-level efforts seek to expand indications for – and access to – nucleos(t)ide analogues (NUC) for chronic hepatitis B (CHB), a corresponding uptick in cases of persistent viremia is expected. Persistent hepatitis B virus (HBV) viremia occurs in nearly 20% of cases and is defined as detectable HBV DNA despite 48 weeks of uninterrupted therapy. Its significance is underlined by two interrelated observations: HBV DNA correlates with future risk of liver-related complications, whereas viral suppression with therapy decreases this risk. A defined clinical approach to persistent HBV viremia is needed, as a paucity of literature on this topic limits current guidance.
Described here are four cases of persistent HBV viremia, who were effectively treated with fixed dose combination medications: emtricitabine-tenofovir disoproxil fumarate (FTC-TDF) or emtricitabine-tenofovir alafenamide (FTC-TAF).
Case Description/
Methods: Included cases have CHB without HIV and varying degrees of underlying liver fibrosis (Table 1-A). Two cases have cirrhosis including one with HCC, which all predated FTC-TDF or FTC-TAF therapy. HBV viremia persisted despite a minimum 1-year course of NUC monotherapy across cases, though baseline HBV DNA levels differed them. Different NUC combinations and sequences were universally unsuccessful (Table 1-B). Genetic profiles were obtained, showing variable resistance patterns.
Response to antiviral therapies is depicted (Figure 1). FTC-TDF was prescribed in three cases, while FTC-TAF was provided in the remaining case. Both led to complete viral suppression within 48 weeks of therapy. Non-invasive tests show stability or regression of underlying fibrosis following therapy. Neither de novo HCC nor virological breakthroughs have been seen after long-term follow-up (range of 1-11 years). Similarly, medication-related adverse events have not occurred with either therapy.
Discussion: Persistent HBV viremia is an understudied problem without a defined treatment pathway. As first reported here, FTC-TDF or FTC-TAF can effectively gain virological control in these cases. FTC is a NUC commonly incorporated into HIV prophylactic and treatment regimens, though it also shows antiviral activity against HBV. It is commercially available and readily prescribed in fixed-dose combination tablets. Thus, FTC-TDF and FTC-TAF are attractive options for persistent HBV viremia. Larger scale studies are warranted to validate these findings and inform future treatment algorithms for persistent HBV viremia.

Figure: Table 1A-B. Patient characteristics. Table 1-A summarizes pertinent medical and viral features of each case. This includes clinical information that predated initiation of either FTC-TDF or FTC-TDF. Table 1-B details each case's antiviral treatment regimens. Abbreviations: BCP mutations (Basal Core Mutations); ETV (Entecavir); F3-F4 (Fibrosis stage 3-4; FIB-4 (Fibrosis-4 Score); FTC (Emtricitabine); HBeAb (HBV e Antibody); HBeAg (HBV e Antigen); HBV (Hepatitis B Virus); HCC (Hepatocellular Carcinoma); HIV (Human Immunodeficiency Virus); HCV (Hepatitis C Virus); HDV (Hepatitis Delta Virus); LSM (Liver Sitffness Measurement); N/A (not applicable); TAF (Tenofovir Alafenamide); TDF (Tenofovir Disoproxil Fumarate).

Figure: Figure 1. Viral Response to Treatment over Time. The virological kinetics for each case is graphically depicted with an accompanying treatment table. The cases are color coordinated: Case 1 (red), Case 2 (purple), Case 3 (green), and Case 4 (blue). On the graph, year zero (0) represents the first recorded HBV DNA level in the EMR for each case. The preceding antiviral therapies received before this time point (year 0) are listed in the table without a corresponding HBV DNA level. For case 1, the patient's index HBV DNA level was significantly higher than the other cases (170,000,000 IU/mL) and thus is illustrated by a dotted red line. Note that while all cases have received FTC-TDF or FTC-TAF for different durations, they are all still presently prescribed these medications as indicated in the table. Importantly, HBV DNA levels were consistently elevated with other NUC therapies across all cases, then became undetectable shortly after initiation of either FTC-TDF or FTC-TAF. Abbreviations: EMR (electronic medical records); ETV (Entecavir); FTC-TAF (Emtricitabine-Tenofovir Alafenamide); FTC-TDF (Emtricitabine-Tenofovir Disoproxil Fumarate); IU/mL (international units per milliliter); LDT (Telbivudine); LMV (Lamivudine); NUC (Nucleos(t)ide Analogues).
Disclosures:
Peter Block indicated no relevant financial relationships.
Ceena Chandrabos indicated no relevant financial relationships.
Viviana Figueroa-Diaz indicated no relevant financial relationships.
Linda Law indicated no relevant financial relationships.
Peter D. Block, MD, MSc, Ceena Chandrabos, MD, Viviana Figueroa-Diaz, MD, Linda Law, MD. P3905 - An Old Problem With a Re-purposed Solution: Novel Application of Emtricitabine-Tenofovir to Treat Persistent HBV Viremia, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
