Monday Poster Session
Category: Liver
P3901 - Unexpected Harvest: Clinical Insights from Three Cases of Amanita Toxicity
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- KH
Kelly Hu, MD
Stanford Health Care
Stanford, CA
Presenting Author(s)
Award: ACG Presidential Poster Award
Kelly Hu, MD1, Marilyn Ndukwe, MD1, Raúl Vázquez-Reyes, MD1, Lynne Martin, MD1, Todd Mitchell, MD2, Paul Kwo, MD1
1Stanford Health Care, Stanford, CA; 2Dominican Hospital, Santa Cruz, CA
Introduction: Unintended ingestion of amatoxin-containing mushrooms is a rare cause of acute liver failure (ALF). Here we present cases of three patients transferred to our academic tertiary care center 48 hours after suspected ingestion of Amanita phalloides.
Case Description/
Methods: Patient A was a 32-year-old healthy man. He reported nausea and diarrhea but was otherwise asymptomatic with normal physical exam and liver ultrasound. Liver enzymes peaked on admission (Table 1A). He was treated with NAC with improvement in liver enzymes and was discharged on hospital day (HD) 3.
Patient B was a 32-year-old man with active alcohol and methamphetamine use. He presented with ALF (grade 2 encephalopathy, elevated INR) and significant liver injury (Table 1B). Imaging confirmed heterogeneous liver enhancement consistent with acute hepatitis. He received NAC, octreotide, activated charcoal, penicillin G, and underwent placement of percutaneous cholecystostomy tube on HD 1. By HD 2 his transaminases and INR were improving. Silibinin was started in lieu of penicillin for a 96-hour course. He was discharged on HD 13.
Patient C was a 37-year-old healthy man who presented with nausea and normal physical exam, though significant liver injury (Table 1C) that rapidly progressed to ALF by the end of HD 1. Imaging revealed diffuse hepatic hypoenhancement consistent with necrosis later confirmed on liver biopsy. He was treated similarly to Patient B, however developed several complications including hemoperitoneum following cholecystostomy, small bowel obstruction from activated charcoal, and amatoxin-mediated kidney failure. He ultimately recovered with supportive care and was discharged on dialysis on HD 32 with recovery of renal function by day 100.
Discussion: Our cases demonstrate the spectrum of illness that may result from amatoxin poisoning, ranging from mild hepatitis to multi-organ failure. Treatment is multifaceted, targeting hepatocyte protection (NAC), reducing hepatocyte uptake of amatoxin (penicillin, silibinin), and disrupting enterohepatic recirculation of amatoxin (octreotide, charcoal). Biliary drainage via cholecystostomy is a potential novel method for amatoxin removal from the enterohepatic circuit and should be reserved for situations in which silibinin is unavailable or clinical progression is rapid. Although there is potential for complications, particularly in coagulopathic patients, it should be considered against the risk for progressive ALF requiring liver transplant.
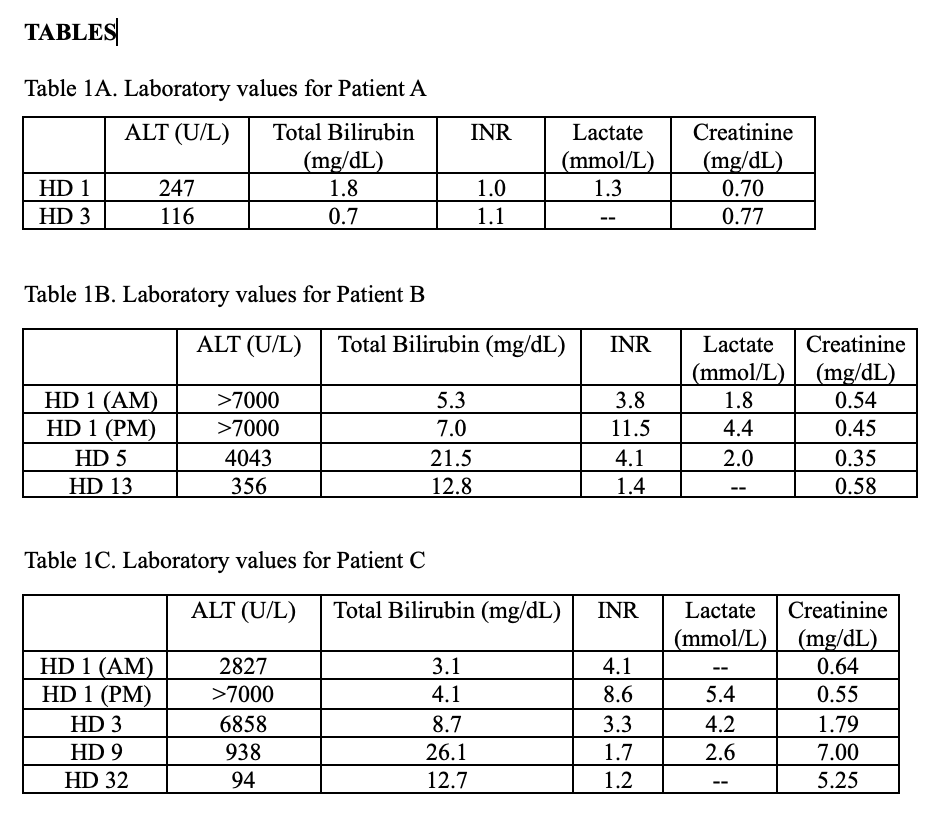
Figure: Laboratory values for Patients A, B, and C.
Disclosures:
Kelly Hu indicated no relevant financial relationships.
Marilyn Ndukwe indicated no relevant financial relationships.
Raúl Vázquez-Reyes indicated no relevant financial relationships.
Lynne Martin indicated no relevant financial relationships.
Todd Mitchell indicated no relevant financial relationships.
Paul Kwo: 89Bio – Grant/Research Support. Akero – Grant/Research Support. Aligos – Consultant. Arbutus – Consultant. Ausper Bio – Consultant, Grant/Research Support. Durect – Consultant, Stock Options. Gilead – Consultant, Grant/Research Support. Glaxo Smith Kline – Consultant. Inventiva – Consultant, Grant/Research Support. Madrigal – Grant/Research Support. Mallinckrodt – Consultant. Novo Nordisk – Consultant, Grant/Research Support. Precision Biosciences – Consultant. Salix – Consultant, Grant/Research Support. Takeda – Grant/Research Support. Tune Therapeutics – Consultant. Ultragenyx – Grant/Research Support.
Kelly Hu, MD1, Marilyn Ndukwe, MD1, Raúl Vázquez-Reyes, MD1, Lynne Martin, MD1, Todd Mitchell, MD2, Paul Kwo, MD1. P3901 - Unexpected Harvest: Clinical Insights from Three Cases of Amanita Toxicity, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Kelly Hu, MD1, Marilyn Ndukwe, MD1, Raúl Vázquez-Reyes, MD1, Lynne Martin, MD1, Todd Mitchell, MD2, Paul Kwo, MD1
1Stanford Health Care, Stanford, CA; 2Dominican Hospital, Santa Cruz, CA
Introduction: Unintended ingestion of amatoxin-containing mushrooms is a rare cause of acute liver failure (ALF). Here we present cases of three patients transferred to our academic tertiary care center 48 hours after suspected ingestion of Amanita phalloides.
Case Description/
Methods: Patient A was a 32-year-old healthy man. He reported nausea and diarrhea but was otherwise asymptomatic with normal physical exam and liver ultrasound. Liver enzymes peaked on admission (Table 1A). He was treated with NAC with improvement in liver enzymes and was discharged on hospital day (HD) 3.
Patient B was a 32-year-old man with active alcohol and methamphetamine use. He presented with ALF (grade 2 encephalopathy, elevated INR) and significant liver injury (Table 1B). Imaging confirmed heterogeneous liver enhancement consistent with acute hepatitis. He received NAC, octreotide, activated charcoal, penicillin G, and underwent placement of percutaneous cholecystostomy tube on HD 1. By HD 2 his transaminases and INR were improving. Silibinin was started in lieu of penicillin for a 96-hour course. He was discharged on HD 13.
Patient C was a 37-year-old healthy man who presented with nausea and normal physical exam, though significant liver injury (Table 1C) that rapidly progressed to ALF by the end of HD 1. Imaging revealed diffuse hepatic hypoenhancement consistent with necrosis later confirmed on liver biopsy. He was treated similarly to Patient B, however developed several complications including hemoperitoneum following cholecystostomy, small bowel obstruction from activated charcoal, and amatoxin-mediated kidney failure. He ultimately recovered with supportive care and was discharged on dialysis on HD 32 with recovery of renal function by day 100.
Discussion: Our cases demonstrate the spectrum of illness that may result from amatoxin poisoning, ranging from mild hepatitis to multi-organ failure. Treatment is multifaceted, targeting hepatocyte protection (NAC), reducing hepatocyte uptake of amatoxin (penicillin, silibinin), and disrupting enterohepatic recirculation of amatoxin (octreotide, charcoal). Biliary drainage via cholecystostomy is a potential novel method for amatoxin removal from the enterohepatic circuit and should be reserved for situations in which silibinin is unavailable or clinical progression is rapid. Although there is potential for complications, particularly in coagulopathic patients, it should be considered against the risk for progressive ALF requiring liver transplant.

Figure: Laboratory values for Patients A, B, and C.
Disclosures:
Kelly Hu indicated no relevant financial relationships.
Marilyn Ndukwe indicated no relevant financial relationships.
Raúl Vázquez-Reyes indicated no relevant financial relationships.
Lynne Martin indicated no relevant financial relationships.
Todd Mitchell indicated no relevant financial relationships.
Paul Kwo: 89Bio – Grant/Research Support. Akero – Grant/Research Support. Aligos – Consultant. Arbutus – Consultant. Ausper Bio – Consultant, Grant/Research Support. Durect – Consultant, Stock Options. Gilead – Consultant, Grant/Research Support. Glaxo Smith Kline – Consultant. Inventiva – Consultant, Grant/Research Support. Madrigal – Grant/Research Support. Mallinckrodt – Consultant. Novo Nordisk – Consultant, Grant/Research Support. Precision Biosciences – Consultant. Salix – Consultant, Grant/Research Support. Takeda – Grant/Research Support. Tune Therapeutics – Consultant. Ultragenyx – Grant/Research Support.
Kelly Hu, MD1, Marilyn Ndukwe, MD1, Raúl Vázquez-Reyes, MD1, Lynne Martin, MD1, Todd Mitchell, MD2, Paul Kwo, MD1. P3901 - Unexpected Harvest: Clinical Insights from Three Cases of Amanita Toxicity, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

