Monday Poster Session
Category: Liver
P3891 - Aloe Vera-Induced Liver Injury with Autoimmune Features
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Simran Joshi, MD
Yale New Haven Health, Bridgeport Hospital
Bridgeport, CT
Presenting Author(s)
Kamal Kandel, MD1, Simran Joshi, MD1, George M. Hanna, MD1, Kusum Sharma, MD2, Armando Dominguez Diaz, MD1, Marie Robert, MD2
1Yale New Haven Health, Bridgeport Hospital, Bridgeport, CT; 2Yale New Haven Health, New Heaven, CT
Introduction: The Centers for Disease Control and Prevention reports that 74% of adults take herbal and dietary supplements (HDS), with 55% being regular users. HDS products are not subject to rigorous safety evaluations or regulatory processes. Data from the Drug-Induced Liver Injury Network suggest that HDS account for approximately 16% of drug-induced liver injury cases, with certain supplements posing a higher risk. Aloe vera is a widely used herbal remedy traditionally used for wound healing, anti-inflammatory effects, and gastrointestinal relief. While generally considered safe, here we describe a case of aloe vera-induced liver injury with autoimmune features.
Case Description/
Methods: A 42-year-old Ecuadorian woman with hypertension and previous cholecystectomy presented with one day of jaundice. The patient reported intermittent right upper quadrant pain for one year, for which she used aloe vera as a remedy. She would remove the outer leaf, blend the inner gel with liquid and consume the mixture. She used it occasionally, but began using it regularly about one month prior to presentation. Her last dose was one week prior to presentation. On examination, she was icteric. Lab abnormalities included total bilirubin 8.4, direct bilirubin 5.3, ALT 1261, AST 1655, ALP 266 and INR 1.36. These values peaked on day 2-3 and then trended downward without intervention (Figure 1). Autoimmune markers were positive with IgG 2104, ANA 1:320 and F-Actin 47. An extensive workup was otherwise unremarkable. Liver biopsy (Figure 2) was consistent with autoimmune hepatitis (AIH) versus drug-induced AIH given the improvement in liver enzymes without intervention. The patient was started on prednisone on day 9 and azathioprine on day 28. The liver enzymes normalized on day 54.
Discussion: Our case appears to be a rare instance of aloe vera-induced liver injury with autoimmune features. Suspected mechanisms of aloe vera-induced liver injury are unclear but may include both direct hepatotoxicity and idiosyncratic injury. The pattern of injury is typically hepatocellular with onset varying from 3 to 24 weeks. Autoimmune features are rare. Management includes discontinuation of the offending agent and symptomatic treatment. This patient was treated for AIH given the degree of liver injury on biopsy. Given the prevalence of HDS use and the potential for hepatotoxicity, a high index of suspicion and a detailed history, including the use of HDS, are crucial in identifying otherwise hidden etiologies of acute hepatitis.
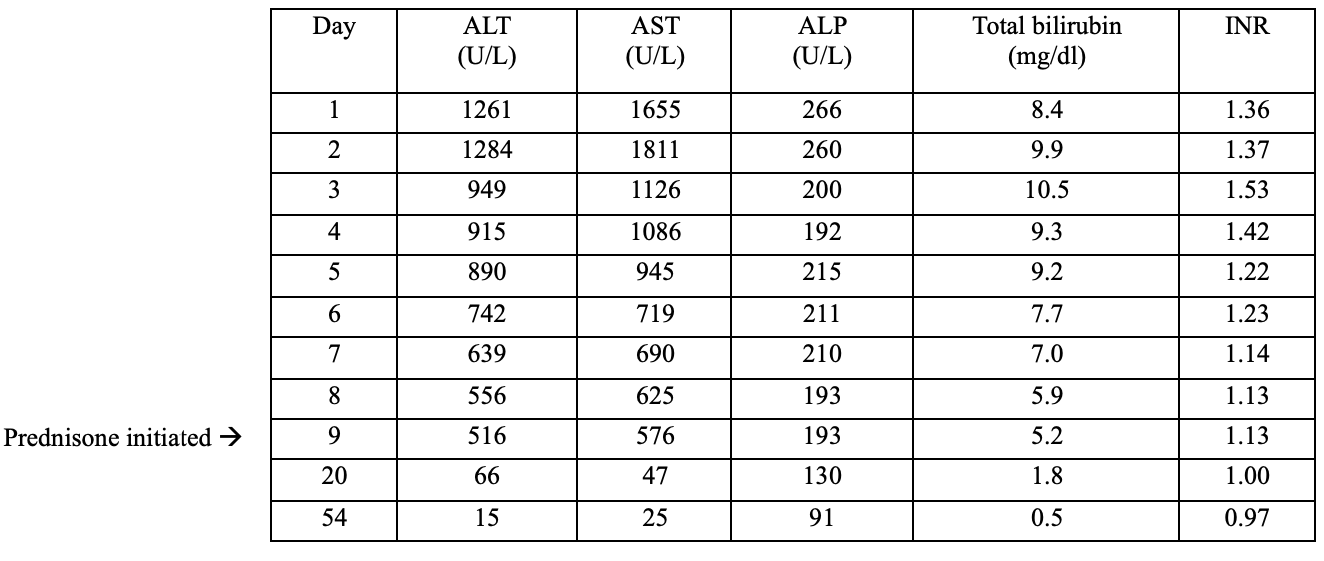
Figure: Figure 1: The trend of liver function test from day 1 to day 54
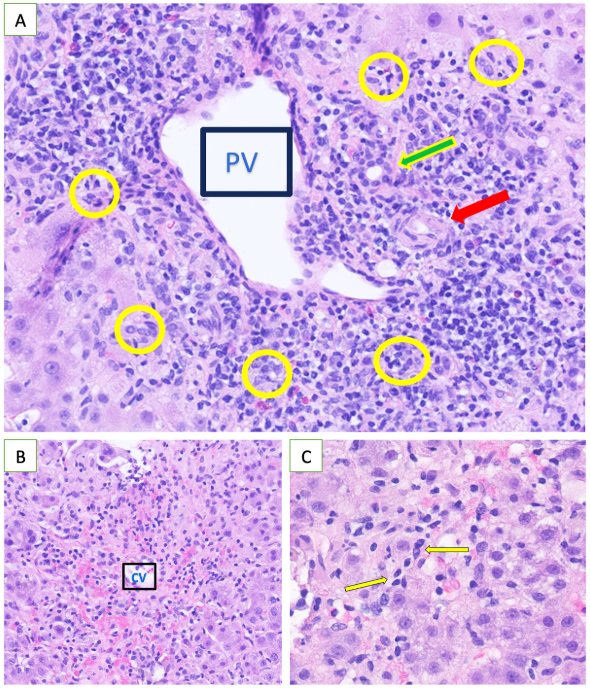
Figure: Figure 2: Liver biopsy
A: Marked portal and interface hepatitis. Portal vein (PV). Bile duct (green arrow), Hepatic artery (red arrow), Interface zone (yellow ovals).
B: Central vein (CV) with surrounding inflammation (lymphocytes, plasma cells and macrophages) and hepatocyte dropout.
C: Higher magnification of peri-central zone with inflammation including plasma cells (yellow arrows).
Disclosures:
Kamal Kandel indicated no relevant financial relationships.
Simran Joshi indicated no relevant financial relationships.
George Hanna indicated no relevant financial relationships.
Kusum Sharma indicated no relevant financial relationships.
Armando Dominguez Diaz indicated no relevant financial relationships.
Marie Robert indicated no relevant financial relationships.
Kamal Kandel, MD1, Simran Joshi, MD1, George M. Hanna, MD1, Kusum Sharma, MD2, Armando Dominguez Diaz, MD1, Marie Robert, MD2. P3891 - Aloe Vera-Induced Liver Injury with Autoimmune Features, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Yale New Haven Health, Bridgeport Hospital, Bridgeport, CT; 2Yale New Haven Health, New Heaven, CT
Introduction: The Centers for Disease Control and Prevention reports that 74% of adults take herbal and dietary supplements (HDS), with 55% being regular users. HDS products are not subject to rigorous safety evaluations or regulatory processes. Data from the Drug-Induced Liver Injury Network suggest that HDS account for approximately 16% of drug-induced liver injury cases, with certain supplements posing a higher risk. Aloe vera is a widely used herbal remedy traditionally used for wound healing, anti-inflammatory effects, and gastrointestinal relief. While generally considered safe, here we describe a case of aloe vera-induced liver injury with autoimmune features.
Case Description/
Methods: A 42-year-old Ecuadorian woman with hypertension and previous cholecystectomy presented with one day of jaundice. The patient reported intermittent right upper quadrant pain for one year, for which she used aloe vera as a remedy. She would remove the outer leaf, blend the inner gel with liquid and consume the mixture. She used it occasionally, but began using it regularly about one month prior to presentation. Her last dose was one week prior to presentation. On examination, she was icteric. Lab abnormalities included total bilirubin 8.4, direct bilirubin 5.3, ALT 1261, AST 1655, ALP 266 and INR 1.36. These values peaked on day 2-3 and then trended downward without intervention (Figure 1). Autoimmune markers were positive with IgG 2104, ANA 1:320 and F-Actin 47. An extensive workup was otherwise unremarkable. Liver biopsy (Figure 2) was consistent with autoimmune hepatitis (AIH) versus drug-induced AIH given the improvement in liver enzymes without intervention. The patient was started on prednisone on day 9 and azathioprine on day 28. The liver enzymes normalized on day 54.
Discussion: Our case appears to be a rare instance of aloe vera-induced liver injury with autoimmune features. Suspected mechanisms of aloe vera-induced liver injury are unclear but may include both direct hepatotoxicity and idiosyncratic injury. The pattern of injury is typically hepatocellular with onset varying from 3 to 24 weeks. Autoimmune features are rare. Management includes discontinuation of the offending agent and symptomatic treatment. This patient was treated for AIH given the degree of liver injury on biopsy. Given the prevalence of HDS use and the potential for hepatotoxicity, a high index of suspicion and a detailed history, including the use of HDS, are crucial in identifying otherwise hidden etiologies of acute hepatitis.

Figure: Figure 1: The trend of liver function test from day 1 to day 54

Figure: Figure 2: Liver biopsy
A: Marked portal and interface hepatitis. Portal vein (PV). Bile duct (green arrow), Hepatic artery (red arrow), Interface zone (yellow ovals).
B: Central vein (CV) with surrounding inflammation (lymphocytes, plasma cells and macrophages) and hepatocyte dropout.
C: Higher magnification of peri-central zone with inflammation including plasma cells (yellow arrows).
Disclosures:
Kamal Kandel indicated no relevant financial relationships.
Simran Joshi indicated no relevant financial relationships.
George Hanna indicated no relevant financial relationships.
Kusum Sharma indicated no relevant financial relationships.
Armando Dominguez Diaz indicated no relevant financial relationships.
Marie Robert indicated no relevant financial relationships.
Kamal Kandel, MD1, Simran Joshi, MD1, George M. Hanna, MD1, Kusum Sharma, MD2, Armando Dominguez Diaz, MD1, Marie Robert, MD2. P3891 - Aloe Vera-Induced Liver Injury with Autoimmune Features, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
