Monday Poster Session
Category: Liver
P3879 - Subacute Liver Injury Leading to Transplant in a Patient Taking Venus Flytrap Extract Supplements
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Lauren A. Lyssy, DO (she/her/hers)
The University of Tennessee Health Science Center
Fort Worth, TX
Presenting Author(s)
Lauren A. Lyssy, DO1, Jeff A. Taclob, MD1, Satheesh Nair, MD2
1The University of Tennessee Health Science Center, Memphis, TN; 2Methodist LeBonheur Healthcare, Memphis, TN
Introduction: Dietary supplement (DS) use is a multi-billion-dollar global industry. It is estimated that more than 50% of Americans take DS, with age, income, and education increasing use. A supplement containing Venus flytrap extract (VFE) is available online and marketed as an immune modulator which selectively targets abnormal cells in the human body. Here, we present a case of subacute liver injury necessitating liver transplant in a patient taking VFE supplements.
Case Description/
Methods: A 68-year-old female was transferred from an outside hospital for evaluation of 1 week of jaundice and malaise. She had no significant past medical history and did not take any prescribed medications. Of note, she did start taking a VFE supplement 7 weeks before the onset of jaundice for “immune support.” She denied abdominal pain, pruritus, fever, or rash. On exam, she was alert and oriented x3 with jaundice and scleral icterus present. Lab work was significant for AST 822, ALT 1,124, ALP 220, total bilirubin 21.3, platelets 88,000, INR 2.6, and ferritin 4,100. Serologic evaluation was negative for viral and autoimmune hepatitis, hemochromatosis, Wilson’s disease, EBV, and CMV infections. CT abdomen triple phase showed a shrunken, nodular liver with patent hepatic and portal vasculature, unremarkable bile ducts, and trace ascites. Liver biopsy on hospital day 1 showed fulminant hepatitis of unclear etiology, with residual viable liver parenchyma in 15% of sampled cores (Figure 1). Subacute liver injury was attributed to VFE supplement use. Due to a lack of significant clinical improvement, the patient was listed for liver transplant with a MELD score of 32 and underwent orthotopic liver transplant (OLT) on hospital day 7.
Discussion: To the best of our knowledge, this is the first case of liver injury associated with VFE supplement use. The mechanism of injury is unclear, but may be related to naturally occurring, potentially hepatotoxic, compounds in the Venus flytrap plant – specifically, droserone, hydroplumbagin, and formic acid. On CT imaging, the liver contour was nodular due to the collapse of hepatic parenchyma. Her ferritin was also significantly elevated due to fulminant hepatitis and the release of ferritin from damaged hepatocytes. This case emphasizes the possibility of severe liver injury in patients taking any DS, even those marketed as non-toxic and supportive of hepatic function.
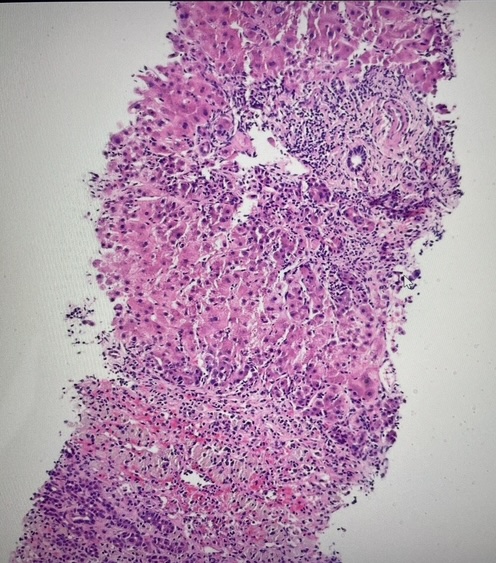
Figure: Figure 1. Liver biopsy showing fulminant hepatitis (acute hepatocellular necrosis) of unclear etiology.
Disclosures:
Lauren Lyssy indicated no relevant financial relationships.
Jeff Taclob indicated no relevant financial relationships.
Satheesh Nair indicated no relevant financial relationships.
Lauren A. Lyssy, DO1, Jeff A. Taclob, MD1, Satheesh Nair, MD2. P3879 - Subacute Liver Injury Leading to Transplant in a Patient Taking Venus Flytrap Extract Supplements, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1The University of Tennessee Health Science Center, Memphis, TN; 2Methodist LeBonheur Healthcare, Memphis, TN
Introduction: Dietary supplement (DS) use is a multi-billion-dollar global industry. It is estimated that more than 50% of Americans take DS, with age, income, and education increasing use. A supplement containing Venus flytrap extract (VFE) is available online and marketed as an immune modulator which selectively targets abnormal cells in the human body. Here, we present a case of subacute liver injury necessitating liver transplant in a patient taking VFE supplements.
Case Description/
Methods: A 68-year-old female was transferred from an outside hospital for evaluation of 1 week of jaundice and malaise. She had no significant past medical history and did not take any prescribed medications. Of note, she did start taking a VFE supplement 7 weeks before the onset of jaundice for “immune support.” She denied abdominal pain, pruritus, fever, or rash. On exam, she was alert and oriented x3 with jaundice and scleral icterus present. Lab work was significant for AST 822, ALT 1,124, ALP 220, total bilirubin 21.3, platelets 88,000, INR 2.6, and ferritin 4,100. Serologic evaluation was negative for viral and autoimmune hepatitis, hemochromatosis, Wilson’s disease, EBV, and CMV infections. CT abdomen triple phase showed a shrunken, nodular liver with patent hepatic and portal vasculature, unremarkable bile ducts, and trace ascites. Liver biopsy on hospital day 1 showed fulminant hepatitis of unclear etiology, with residual viable liver parenchyma in 15% of sampled cores (Figure 1). Subacute liver injury was attributed to VFE supplement use. Due to a lack of significant clinical improvement, the patient was listed for liver transplant with a MELD score of 32 and underwent orthotopic liver transplant (OLT) on hospital day 7.
Discussion: To the best of our knowledge, this is the first case of liver injury associated with VFE supplement use. The mechanism of injury is unclear, but may be related to naturally occurring, potentially hepatotoxic, compounds in the Venus flytrap plant – specifically, droserone, hydroplumbagin, and formic acid. On CT imaging, the liver contour was nodular due to the collapse of hepatic parenchyma. Her ferritin was also significantly elevated due to fulminant hepatitis and the release of ferritin from damaged hepatocytes. This case emphasizes the possibility of severe liver injury in patients taking any DS, even those marketed as non-toxic and supportive of hepatic function.

Figure: Figure 1. Liver biopsy showing fulminant hepatitis (acute hepatocellular necrosis) of unclear etiology.
Disclosures:
Lauren Lyssy indicated no relevant financial relationships.
Jeff Taclob indicated no relevant financial relationships.
Satheesh Nair indicated no relevant financial relationships.
Lauren A. Lyssy, DO1, Jeff A. Taclob, MD1, Satheesh Nair, MD2. P3879 - Subacute Liver Injury Leading to Transplant in a Patient Taking Venus Flytrap Extract Supplements, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

