Monday Poster Session
Category: Liver
P3834 - Therapeutic Risk in a Compromised Liver: Resmetirom-Related Hepatotoxicity in Alcoholic Liver Disease
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Charitha Karanam Ramapathy, MD (she/her/hers)
UAB Montgomery
Montgomery, AL
Presenting Author(s)
Charitha Karanam Ramapathy, MD1, Sree Manasa PuttamReddy, MD2, Yousif Elmofti, MD, FACG1
1UAB Montgomery, Montgomery, AL; 2University of Alabama, Montgomery, AL
Introduction: Drug-induced liver injury (DILI) is a form of hepatotoxicity resulting from an idiosyncratic or dose-dependent adverse reaction to pharmacologic agents. It remains relatively rare, with an estimated incidence of 14–19 cases per 100,000 individuals annually, and accounts for less than 1% of all acute liver injury presentations. DILI is an infrequent yet potentially life-threatening adverse drug reaction.
Case Description/
Methods: A 39-year-old female with a history of metabolic dysfunction-associated steatohepatitis (MASH) was admitted for evaluation of abnormal liver function tests. She denied abdominal pain, NSAID or herbal supplement use. She had recently started Resmetirom 80 mg. Physical exam revealed scleral icterus. Labs showed INR 9.53, PT 80.8 seconds, with negative hepatitis panel and normal alcohol, acetaminophen, and salicylate levels. Abdominal CT showed hepatomegaly, hepatic steatosis with fibrosis, and no biliary ductal dilatation. A prior liver biopsy revealed steatohepatitis with stage 3 bridging fibrosis. She was managed conservatively and transferred for liver transplant evaluation. Initially denying alcohol use, she later admitted to drinking 1–2 glasses of wine several times a week, after testing positive for phosphatidylethanol (PEth) at 447. Her hospital course was complicated by respiratory distress and acute anemia requiring intubation and vasopressors. Chest CT showed active extravasation with a large right hemothorax. Despite intervention, her condition deteriorated, and eventually passed away.
Discussion: Resmetirom is a recently approved FDA medication to treat liver fibrosis associated with NASH[nonalcoholic steatohepatitis] with the name being changed to metabolic dysfunction-associated steatohepatitis [MASH]. In instances where acute liver failure develops, immediate transfer to an intensive care setting and prompt referral to a specialized hepatology center for liver transplantation assessment is warranted. In our case, the patient developed acute-on-chronic liver injury within a few weeks of initiating resmetirom therapy. It remains uncertain whether resmetirom directly contributes to drug-induced liver injury (DILI) or if hepatotoxicity may be confined to specific subpopulations, such as those with concurrent alcohol use. Further research is needed to elucidate the hepatotoxic potential of resmetirom and to identify any at-risk patient groups.
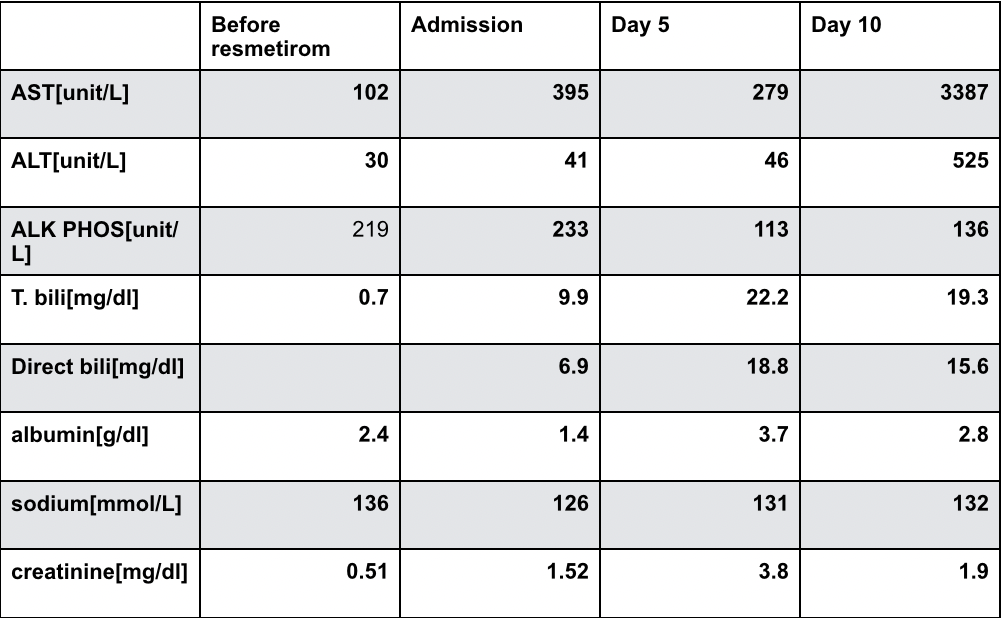
Figure: Table 1 - Laboratory Trends During Hospitalization
Disclosures:
Charitha Karanam Ramapathy indicated no relevant financial relationships.
Sree Manasa PuttamReddy indicated no relevant financial relationships.
Yousif Elmofti indicated no relevant financial relationships.
Charitha Karanam Ramapathy, MD1, Sree Manasa PuttamReddy, MD2, Yousif Elmofti, MD, FACG1. P3834 - Therapeutic Risk in a Compromised Liver: Resmetirom-Related Hepatotoxicity in Alcoholic Liver Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1UAB Montgomery, Montgomery, AL; 2University of Alabama, Montgomery, AL
Introduction: Drug-induced liver injury (DILI) is a form of hepatotoxicity resulting from an idiosyncratic or dose-dependent adverse reaction to pharmacologic agents. It remains relatively rare, with an estimated incidence of 14–19 cases per 100,000 individuals annually, and accounts for less than 1% of all acute liver injury presentations. DILI is an infrequent yet potentially life-threatening adverse drug reaction.
Case Description/
Methods: A 39-year-old female with a history of metabolic dysfunction-associated steatohepatitis (MASH) was admitted for evaluation of abnormal liver function tests. She denied abdominal pain, NSAID or herbal supplement use. She had recently started Resmetirom 80 mg. Physical exam revealed scleral icterus. Labs showed INR 9.53, PT 80.8 seconds, with negative hepatitis panel and normal alcohol, acetaminophen, and salicylate levels. Abdominal CT showed hepatomegaly, hepatic steatosis with fibrosis, and no biliary ductal dilatation. A prior liver biopsy revealed steatohepatitis with stage 3 bridging fibrosis. She was managed conservatively and transferred for liver transplant evaluation. Initially denying alcohol use, she later admitted to drinking 1–2 glasses of wine several times a week, after testing positive for phosphatidylethanol (PEth) at 447. Her hospital course was complicated by respiratory distress and acute anemia requiring intubation and vasopressors. Chest CT showed active extravasation with a large right hemothorax. Despite intervention, her condition deteriorated, and eventually passed away.
Discussion: Resmetirom is a recently approved FDA medication to treat liver fibrosis associated with NASH[nonalcoholic steatohepatitis] with the name being changed to metabolic dysfunction-associated steatohepatitis [MASH]. In instances where acute liver failure develops, immediate transfer to an intensive care setting and prompt referral to a specialized hepatology center for liver transplantation assessment is warranted. In our case, the patient developed acute-on-chronic liver injury within a few weeks of initiating resmetirom therapy. It remains uncertain whether resmetirom directly contributes to drug-induced liver injury (DILI) or if hepatotoxicity may be confined to specific subpopulations, such as those with concurrent alcohol use. Further research is needed to elucidate the hepatotoxic potential of resmetirom and to identify any at-risk patient groups.

Figure: Table 1 - Laboratory Trends During Hospitalization
Disclosures:
Charitha Karanam Ramapathy indicated no relevant financial relationships.
Sree Manasa PuttamReddy indicated no relevant financial relationships.
Yousif Elmofti indicated no relevant financial relationships.
Charitha Karanam Ramapathy, MD1, Sree Manasa PuttamReddy, MD2, Yousif Elmofti, MD, FACG1. P3834 - Therapeutic Risk in a Compromised Liver: Resmetirom-Related Hepatotoxicity in Alcoholic Liver Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
