Monday Poster Session
Category: Liver
P3829 - From Pacific Isles to Liver Trials: Unraveling the Toxic Tale of Kava
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Geraldine Nabeta, MD, MS (she/her/hers)
University of Connecticut Health Center
Farmington, CT
Presenting Author(s)
Award: ACG Presidential Poster Award
Geraldine Nabeta, MD, MS1, Mirghani Mohamed, BS2, Courtney Stead, MD1, Mridula Sree Naagendran, MBBS3, Rishabh Sachdev, MD4
1University of Connecticut Health Center, Farmington, CT; 2University of Connecticut School of Medicine, Farmington, CT; 3University of Connecticut, Farmington, CT; 4University of Connecticut Health, Farmington, CT
Introduction: Kava, a drink consumed across several South Pacific islands, has grown in popularity for its sedative, hypnotic, and muscle relaxant effects. As its use increases, understanding its toxicological profile becomes crucial. A pilot health survey has shown that heavy kava use has been linked to hematological conditions, liver damage, and rashes. Here we present a case of a 20-year-old female patient, with no significant past medical history, admitted with elevated liver enzymes and a diffuse rash in the setting of kava use.
Case Description/
Methods: The patient presented with a five-day history of facial edema and a worsening pruritic, vesiculopustular rash involving her abdomen, extremities, face, and scalp. She had associated symptoms of fevers, chills, and nausea. Prior to presentation, significant changes to her medication history included recent kava tea consumption. On admission, she was febrile to 100.4°F, but otherwise hemodynamically stable. Labs were significant for mild leukocytosis, and anemia, platelets at 194 10³/µL, hepatocellular pattern of liver injury (AST 117 U/L ALT 195 U/L ALP 128 U/L), and total bilirubin 0.4. ESR and CRP elevated. She did not demonstrate evidence of encephalopathy or synthetic dysfunction. Hepatitis panel, HIV, tick-borne panel, and autoimmune panel were all negative. Ultrasound of the right upper quadrant was only significant for mild hepatomegaly.
The patient ultimately underwent a skin biopsy which revealed pathology suspicious for drug-induced/supplement-induced hypersensitivity reactions. She was started on a steroid taper resulting in an improvement in her rash, symptoms and liver enzymes. The timing of symptom onset relative to kava consumption and the exclusion of other causes of acute liver injury, support a diagnosis of drug-induced liver injury (DILI) from Kava.
Discussion: Kava-induced hepatotoxicity results from oxidative stress in hepatocytes. Oftentimes kava results in a hepatocellular pattern of injury that can progress to fulminant liver injury. There have been 30 reported incidences of hepatotoxicity in Europe and four have required transplants. The hepatotoxic potential of kava necessitates caution. This case underscores the warnings issued by health authorities and adds to the growing body of evidence on kava toxicity. Furthermore, this case emphasizes the importance of considering herbal supplements as part of a comprehensive medication history, when evaluating patients with unexplained elevated liver enzymes.
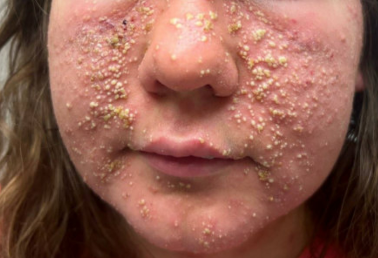
Figure: Extensive vesiculopustular lesions over the nasolabial and cheeks
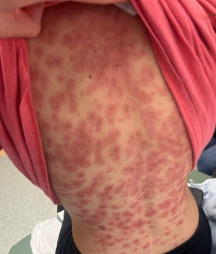
Figure: Diffuse erythematous and maculopapular rash over the entire back
Disclosures:
Geraldine Nabeta indicated no relevant financial relationships.
Mirghani Mohamed indicated no relevant financial relationships.
Courtney Stead indicated no relevant financial relationships.
Mridula Sree Naagendran indicated no relevant financial relationships.
Rishabh Sachdev indicated no relevant financial relationships.
Geraldine Nabeta, MD, MS1, Mirghani Mohamed, BS2, Courtney Stead, MD1, Mridula Sree Naagendran, MBBS3, Rishabh Sachdev, MD4. P3829 - From Pacific Isles to Liver Trials: Unraveling the Toxic Tale of Kava, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Geraldine Nabeta, MD, MS1, Mirghani Mohamed, BS2, Courtney Stead, MD1, Mridula Sree Naagendran, MBBS3, Rishabh Sachdev, MD4
1University of Connecticut Health Center, Farmington, CT; 2University of Connecticut School of Medicine, Farmington, CT; 3University of Connecticut, Farmington, CT; 4University of Connecticut Health, Farmington, CT
Introduction: Kava, a drink consumed across several South Pacific islands, has grown in popularity for its sedative, hypnotic, and muscle relaxant effects. As its use increases, understanding its toxicological profile becomes crucial. A pilot health survey has shown that heavy kava use has been linked to hematological conditions, liver damage, and rashes. Here we present a case of a 20-year-old female patient, with no significant past medical history, admitted with elevated liver enzymes and a diffuse rash in the setting of kava use.
Case Description/
Methods: The patient presented with a five-day history of facial edema and a worsening pruritic, vesiculopustular rash involving her abdomen, extremities, face, and scalp. She had associated symptoms of fevers, chills, and nausea. Prior to presentation, significant changes to her medication history included recent kava tea consumption. On admission, she was febrile to 100.4°F, but otherwise hemodynamically stable. Labs were significant for mild leukocytosis, and anemia, platelets at 194 10³/µL, hepatocellular pattern of liver injury (AST 117 U/L ALT 195 U/L ALP 128 U/L), and total bilirubin 0.4. ESR and CRP elevated. She did not demonstrate evidence of encephalopathy or synthetic dysfunction. Hepatitis panel, HIV, tick-borne panel, and autoimmune panel were all negative. Ultrasound of the right upper quadrant was only significant for mild hepatomegaly.
The patient ultimately underwent a skin biopsy which revealed pathology suspicious for drug-induced/supplement-induced hypersensitivity reactions. She was started on a steroid taper resulting in an improvement in her rash, symptoms and liver enzymes. The timing of symptom onset relative to kava consumption and the exclusion of other causes of acute liver injury, support a diagnosis of drug-induced liver injury (DILI) from Kava.
Discussion: Kava-induced hepatotoxicity results from oxidative stress in hepatocytes. Oftentimes kava results in a hepatocellular pattern of injury that can progress to fulminant liver injury. There have been 30 reported incidences of hepatotoxicity in Europe and four have required transplants. The hepatotoxic potential of kava necessitates caution. This case underscores the warnings issued by health authorities and adds to the growing body of evidence on kava toxicity. Furthermore, this case emphasizes the importance of considering herbal supplements as part of a comprehensive medication history, when evaluating patients with unexplained elevated liver enzymes.

Figure: Extensive vesiculopustular lesions over the nasolabial and cheeks

Figure: Diffuse erythematous and maculopapular rash over the entire back
Disclosures:
Geraldine Nabeta indicated no relevant financial relationships.
Mirghani Mohamed indicated no relevant financial relationships.
Courtney Stead indicated no relevant financial relationships.
Mridula Sree Naagendran indicated no relevant financial relationships.
Rishabh Sachdev indicated no relevant financial relationships.
Geraldine Nabeta, MD, MS1, Mirghani Mohamed, BS2, Courtney Stead, MD1, Mridula Sree Naagendran, MBBS3, Rishabh Sachdev, MD4. P3829 - From Pacific Isles to Liver Trials: Unraveling the Toxic Tale of Kava, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

