Monday Poster Session
Category: Liver
P3815 - Magnetically Guided Capsule Endoscopy for Detecting Esophageal Varices: Systematic Review and Meta-Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- TA
Tareq Alsaleh, MD
Department of Internal Medicine, AdventHealth Orlando
Orlando, FL
Presenting Author(s)
Tareq Alsaleh, MD1, Prachi Mann, MD2, Nouman Shafique, MD1, Bassel Dakkak, MD3, Mohamad Khaled Almujarkesh, MD4, John George, MD4
1Department of Internal Medicine, AdventHealth Orlando, Orlando, FL; 2Department of Internal Medicine, Adventhealth Orlando, Orlando, FL; 3Marshall University Joan C. Edwards School of Medicine, Huntington, WV; 4Department of Gastroenterology and Hepatology, AdventHealth Orlando, Orlando, FL
Introduction: Screening and surveillance for high-risk esophageal varices (EV) is recommended in patients with liver cirrhosis to allow for primary prophylaxis of variceal bleeding. Esophagogastroduodenoscopy (EGD) is the diagnostic modality of choice, but it is an invasive procedure that can lead to sedation-relation complications and low adherence to screening. Magnetically guided capsule endoscopy (MCE) may offer a less invasive, alternative approach. We conducted a systematic review and meta-analysis to evaluate the diagnostic performance of MCE in detection of EV.
Methods: A systematic review of the literature from PubMed, EMBASE, and Scopus was conducted through April 2025 for studies evaluating the diagnostic performance of MCE with EGD as the reference gold-standard. Primary outcomes of interest were sensitivity and specificity. For every study, the number of true positives, true negatives, false positives and false negatives was retrieved and documented. Standard meta-analysis methods were followed using the bivariate random-effects model. Heterogeneity was assessed using the I2% statistic.
Results: Three prospective studies were included, comprising 737 patients. Two studies were multicenter, while one was single center. The participants included 512 (69.5%) males. The investigators were blinded in all three studies. Two studies used detachable strings to assist with the MCE (Table 1).
Reported sensitivity ranged from 73.3% to 96.4% in the individual studies, while specificity ranged from 88% to 100%. The pooled estimate of sensitivity was 90.6% (95% CI: 71%-97.4%; I2=84.9%) and the pooled estimate of specificity was 96% (95% CI: 86%-98.9%; I2=72.5%). Forest plots for sensitivity and specificity are shown in Figure 1.
Subgroup analysis of studies using detachable-string MCE showed pooled estimates of sensitivity of 95.3% (95% CI: 90.1%-97.8%; I2=43%) and specificity of 95.2% (95% CI: 78.8%-99.1%; I2=85.9%). No MCE-related adverse events were reported among the three studies.
Discussion: The results of our meta-analysis suggest that MCE is a highly accurate, safe, and promising diagnostic test for the detection of esophageal varices and may play a practical role in screening and surveillance of EV. Its less invasive nature may also improve adherence to endoscopic follow-up. The use of detachable strings may further enhance the sensitivity of the test. Further large-scale prospective studies are needed to better define its role in evaluating EV and other upper gastrointestinal lesions.
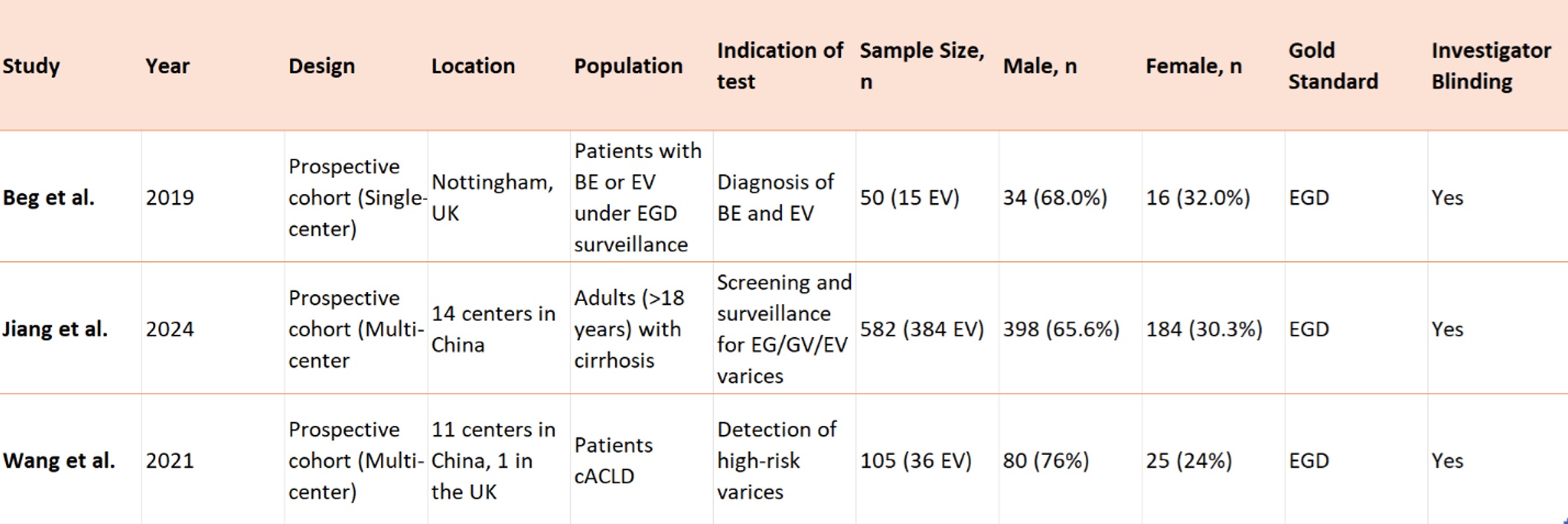
Figure: Table 1. Characteristics of included studies.
Abbreviations: BE, Barrett’s Esophagus; cACLD, compensated Advanced Chronic liver disease; EV, Esophageal Varices; EG varices, Esophagogastric varices; GV, Gastric varices.
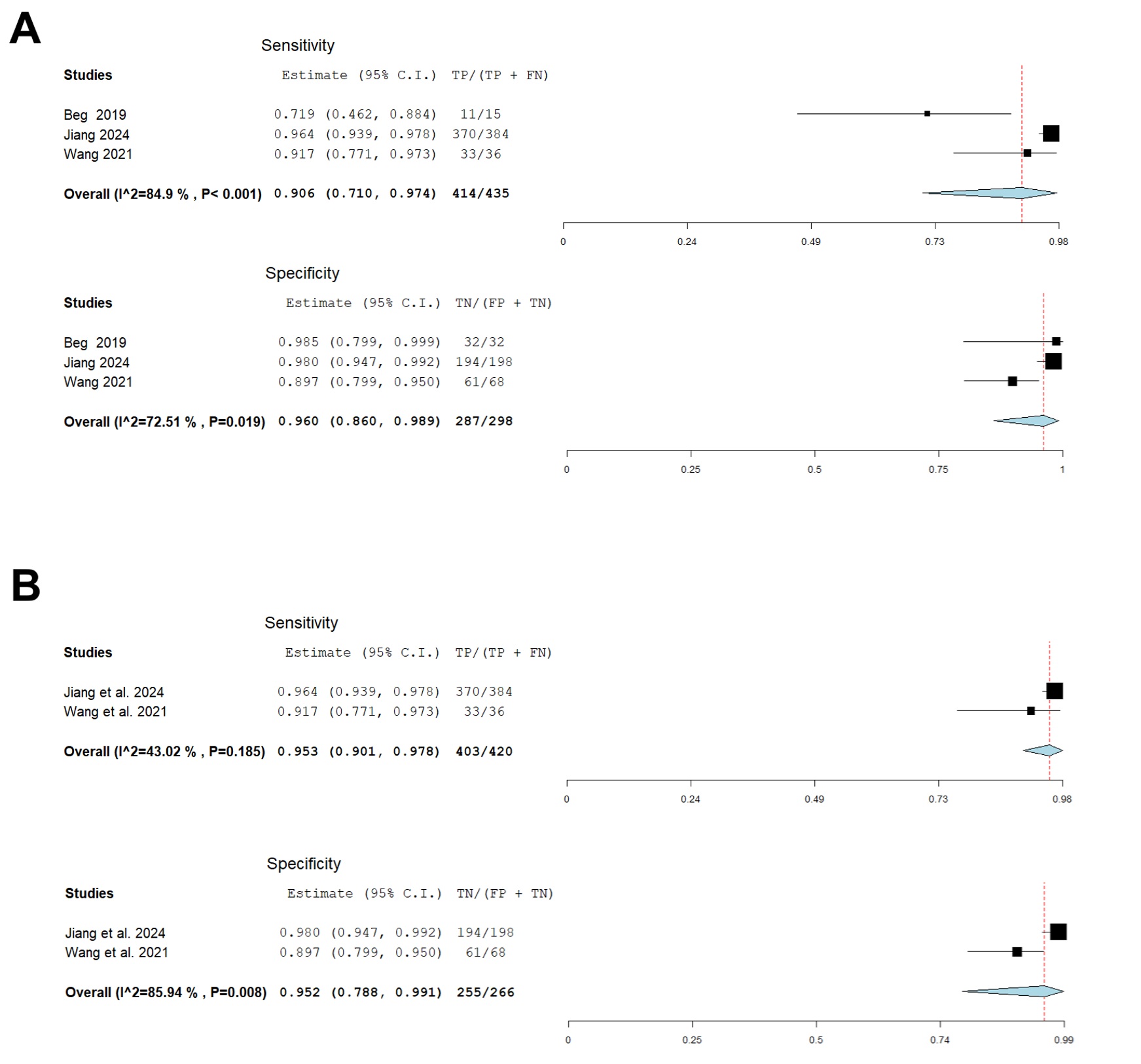
Figure: A – Pooled sensitivity and specificity of MCE for detection of EV.
B – Pooled sensitivity and specificity of detachable-string-MCE for detection of EV
Disclosures:
Tareq Alsaleh indicated no relevant financial relationships.
Prachi Mann indicated no relevant financial relationships.
Nouman Shafique indicated no relevant financial relationships.
Bassel Dakkak indicated no relevant financial relationships.
Mohamad Khaled Almujarkesh indicated no relevant financial relationships.
John George indicated no relevant financial relationships.
Tareq Alsaleh, MD1, Prachi Mann, MD2, Nouman Shafique, MD1, Bassel Dakkak, MD3, Mohamad Khaled Almujarkesh, MD4, John George, MD4. P3815 - Magnetically Guided Capsule Endoscopy for Detecting Esophageal Varices: Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Internal Medicine, AdventHealth Orlando, Orlando, FL; 2Department of Internal Medicine, Adventhealth Orlando, Orlando, FL; 3Marshall University Joan C. Edwards School of Medicine, Huntington, WV; 4Department of Gastroenterology and Hepatology, AdventHealth Orlando, Orlando, FL
Introduction: Screening and surveillance for high-risk esophageal varices (EV) is recommended in patients with liver cirrhosis to allow for primary prophylaxis of variceal bleeding. Esophagogastroduodenoscopy (EGD) is the diagnostic modality of choice, but it is an invasive procedure that can lead to sedation-relation complications and low adherence to screening. Magnetically guided capsule endoscopy (MCE) may offer a less invasive, alternative approach. We conducted a systematic review and meta-analysis to evaluate the diagnostic performance of MCE in detection of EV.
Methods: A systematic review of the literature from PubMed, EMBASE, and Scopus was conducted through April 2025 for studies evaluating the diagnostic performance of MCE with EGD as the reference gold-standard. Primary outcomes of interest were sensitivity and specificity. For every study, the number of true positives, true negatives, false positives and false negatives was retrieved and documented. Standard meta-analysis methods were followed using the bivariate random-effects model. Heterogeneity was assessed using the I2% statistic.
Results: Three prospective studies were included, comprising 737 patients. Two studies were multicenter, while one was single center. The participants included 512 (69.5%) males. The investigators were blinded in all three studies. Two studies used detachable strings to assist with the MCE (Table 1).
Reported sensitivity ranged from 73.3% to 96.4% in the individual studies, while specificity ranged from 88% to 100%. The pooled estimate of sensitivity was 90.6% (95% CI: 71%-97.4%; I2=84.9%) and the pooled estimate of specificity was 96% (95% CI: 86%-98.9%; I2=72.5%). Forest plots for sensitivity and specificity are shown in Figure 1.
Subgroup analysis of studies using detachable-string MCE showed pooled estimates of sensitivity of 95.3% (95% CI: 90.1%-97.8%; I2=43%) and specificity of 95.2% (95% CI: 78.8%-99.1%; I2=85.9%). No MCE-related adverse events were reported among the three studies.
Discussion: The results of our meta-analysis suggest that MCE is a highly accurate, safe, and promising diagnostic test for the detection of esophageal varices and may play a practical role in screening and surveillance of EV. Its less invasive nature may also improve adherence to endoscopic follow-up. The use of detachable strings may further enhance the sensitivity of the test. Further large-scale prospective studies are needed to better define its role in evaluating EV and other upper gastrointestinal lesions.

Figure: Table 1. Characteristics of included studies.
Abbreviations: BE, Barrett’s Esophagus; cACLD, compensated Advanced Chronic liver disease; EV, Esophageal Varices; EG varices, Esophagogastric varices; GV, Gastric varices.

Figure: A – Pooled sensitivity and specificity of MCE for detection of EV.
B – Pooled sensitivity and specificity of detachable-string-MCE for detection of EV
Disclosures:
Tareq Alsaleh indicated no relevant financial relationships.
Prachi Mann indicated no relevant financial relationships.
Nouman Shafique indicated no relevant financial relationships.
Bassel Dakkak indicated no relevant financial relationships.
Mohamad Khaled Almujarkesh indicated no relevant financial relationships.
John George indicated no relevant financial relationships.
Tareq Alsaleh, MD1, Prachi Mann, MD2, Nouman Shafique, MD1, Bassel Dakkak, MD3, Mohamad Khaled Almujarkesh, MD4, John George, MD4. P3815 - Magnetically Guided Capsule Endoscopy for Detecting Esophageal Varices: Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
