Monday Poster Session
Category: Liver
P3797 - Efficacy of Peroxisome Proliferator-Activated Receptor (PPAR) Agonists in the Treatment of Primary Biliary Cholangitis: A Meta-Analysis of Randomized Controlled Trials
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Sabtain Saroya, BS (he/him/his)
College of Medicine, Howard University
Washington, DC
Presenting Author(s)
Sabtain Saroya, BS1, Andrew Canakis, DO2, Umme Ferdaush, MD3, Nuha Mohammed, 4, Emily Gorman, 5, Zurabi Lominadze, MD6, Kirti Shetty, MD7
1College of Medicine, Howard University, Washington, DC; 2Unc chapel hill, Bishopville, MD; 3TidalHealth Peninsula Regional, Salisbury, MD; 4College of Medicine, Howard University, Catonsville, MD; 5University of Maryland Medical System, Catonsville, MD; 6University of Maryland Medical Center, Baltimore, MD; 7University of Maryland School of Medicine, Baltimore, MD
Introduction: Primary biliary cholangitis (PBC) is a chronic autoimmune liver disease marked by progressive bile duct destruction. While ursodeoxycholic acid (UDCA) is first-line therapy, approximately 40% of patients show an incomplete response. Peroxisome proliferator-activated receptor (PPAR) agonists have emerged as promising agents due to their anti-inflammatory and metabolic effects. This meta-analysis evaluates the efficacy of PPAR agonists in improving liver biochemical markers in PBC.
Methods: A systematic search of MEDLINE, Embase, and Scopus was performed to identify randomized controlled trials (RCTs) comparing PPAR agonists to placebo in patients with PBC. Eligible studies (1) were placebo-controlled RCTs, (2) included patients with PBC regardless of disease stage or PPAR agonist dose, and (3) reported pre- and post-treatment values for alkaline phosphatase (ALP), bilirubin, and gamma-glutamyl transferase (GGT). Exclusion criteria included studies without a placebo or control group, non-human studies, trials with no relevant outcomes, and trials with incomplete or withdrawn data. Six trials met these criteria. Follow-up durations ranged from 3 to 24 months. To account for variability in treatment effect due to time, we used post-minus-pre-treatment mean differences and a random-effects model. Standard errors were derived using an assumed correlation coefficient (r = 0.7) between paired values. Pooled estimates were weighted by inverse variance and calculated separately for each biomarker.
Results: PPAR agonists were associated with significant reductions in liver biomarkers compared to placebo. ALP decreased by –87.14 U/L (95% CI: –90.20, –84.08). Bilirubin declined by –1.04 mg/dL (95% CI: –1.37, –0.71). GGT was reduced by –46.90 U/L (95% CI: –51.64, –42.16). All reductions were statistically significant.
Discussion: In placebo-controlled trials, PPAR agonists significantly improved ALP, bilirubin, and GGT levels in patients with PBC. These findings support their potential role as effective adjunctive therapy, particularly for patients with inadequate response to UDCA.
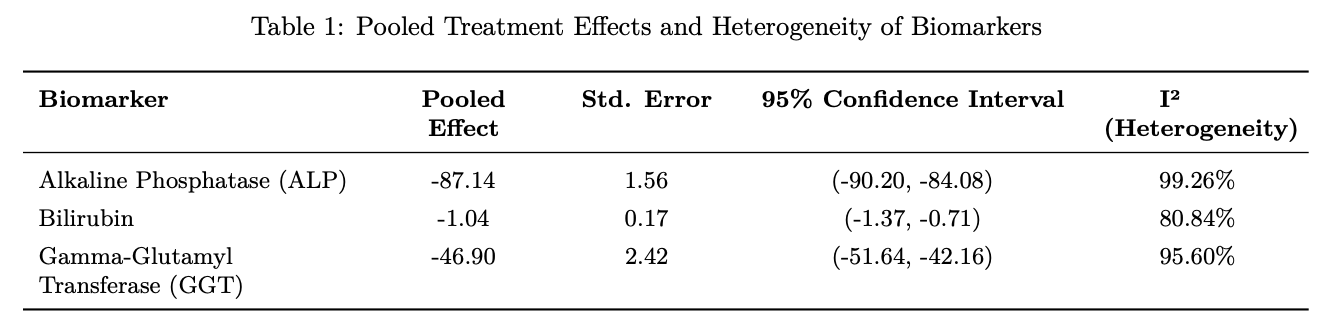
Figure: Figure 1. Pooled effect sizes of PPAR agonists on liver biochemical markers in patients with primary biliary cholangitis (PBC). Biomarkers include ALP, GGT, Bilirubin. Data reflect standardized mean differences with 95% confidence intervals across included randomized controlled trials.
Disclosures:
Sabtain Saroya indicated no relevant financial relationships.
Andrew Canakis indicated no relevant financial relationships.
Umme Ferdaush indicated no relevant financial relationships.
Nuha Mohammed indicated no relevant financial relationships.
Emily Gorman indicated no relevant financial relationships.
Zurabi Lominadze indicated no relevant financial relationships.
Kirti Shetty indicated no relevant financial relationships.
Sabtain Saroya, BS1, Andrew Canakis, DO2, Umme Ferdaush, MD3, Nuha Mohammed, 4, Emily Gorman, 5, Zurabi Lominadze, MD6, Kirti Shetty, MD7. P3797 - Efficacy of Peroxisome Proliferator-Activated Receptor (PPAR) Agonists in the Treatment of Primary Biliary Cholangitis: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1College of Medicine, Howard University, Washington, DC; 2Unc chapel hill, Bishopville, MD; 3TidalHealth Peninsula Regional, Salisbury, MD; 4College of Medicine, Howard University, Catonsville, MD; 5University of Maryland Medical System, Catonsville, MD; 6University of Maryland Medical Center, Baltimore, MD; 7University of Maryland School of Medicine, Baltimore, MD
Introduction: Primary biliary cholangitis (PBC) is a chronic autoimmune liver disease marked by progressive bile duct destruction. While ursodeoxycholic acid (UDCA) is first-line therapy, approximately 40% of patients show an incomplete response. Peroxisome proliferator-activated receptor (PPAR) agonists have emerged as promising agents due to their anti-inflammatory and metabolic effects. This meta-analysis evaluates the efficacy of PPAR agonists in improving liver biochemical markers in PBC.
Methods: A systematic search of MEDLINE, Embase, and Scopus was performed to identify randomized controlled trials (RCTs) comparing PPAR agonists to placebo in patients with PBC. Eligible studies (1) were placebo-controlled RCTs, (2) included patients with PBC regardless of disease stage or PPAR agonist dose, and (3) reported pre- and post-treatment values for alkaline phosphatase (ALP), bilirubin, and gamma-glutamyl transferase (GGT). Exclusion criteria included studies without a placebo or control group, non-human studies, trials with no relevant outcomes, and trials with incomplete or withdrawn data. Six trials met these criteria. Follow-up durations ranged from 3 to 24 months. To account for variability in treatment effect due to time, we used post-minus-pre-treatment mean differences and a random-effects model. Standard errors were derived using an assumed correlation coefficient (r = 0.7) between paired values. Pooled estimates were weighted by inverse variance and calculated separately for each biomarker.
Results: PPAR agonists were associated with significant reductions in liver biomarkers compared to placebo. ALP decreased by –87.14 U/L (95% CI: –90.20, –84.08). Bilirubin declined by –1.04 mg/dL (95% CI: –1.37, –0.71). GGT was reduced by –46.90 U/L (95% CI: –51.64, –42.16). All reductions were statistically significant.
Discussion: In placebo-controlled trials, PPAR agonists significantly improved ALP, bilirubin, and GGT levels in patients with PBC. These findings support their potential role as effective adjunctive therapy, particularly for patients with inadequate response to UDCA.

Figure: Figure 1. Pooled effect sizes of PPAR agonists on liver biochemical markers in patients with primary biliary cholangitis (PBC). Biomarkers include ALP, GGT, Bilirubin. Data reflect standardized mean differences with 95% confidence intervals across included randomized controlled trials.
Disclosures:
Sabtain Saroya indicated no relevant financial relationships.
Andrew Canakis indicated no relevant financial relationships.
Umme Ferdaush indicated no relevant financial relationships.
Nuha Mohammed indicated no relevant financial relationships.
Emily Gorman indicated no relevant financial relationships.
Zurabi Lominadze indicated no relevant financial relationships.
Kirti Shetty indicated no relevant financial relationships.
Sabtain Saroya, BS1, Andrew Canakis, DO2, Umme Ferdaush, MD3, Nuha Mohammed, 4, Emily Gorman, 5, Zurabi Lominadze, MD6, Kirti Shetty, MD7. P3797 - Efficacy of Peroxisome Proliferator-Activated Receptor (PPAR) Agonists in the Treatment of Primary Biliary Cholangitis: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
