Monday Poster Session
Category: Liver
P3753 - Safety Profile of Placebo in Patients With Primary Biliary Cirrhosis: A Systematic Review and Meta-Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Aalam Sohal, MD
Creighton University School of Medicine
Phoenix, AZ
Presenting Author(s)
Sampada Bhasker, MBBS1, Aalam Sohal, MD2, Vikash Karmani, MBBS3, Vikash Kumar, MD4, Divyesh Sejpal, MD, MS2
1Punjab Institute of Medical Sciences, Jalandhar, Punjab, India; 2Creighton University School of Medicine, Phoenix, AZ; 3Jinnah Sindh Medical University, Karachi, Sindh, Pakistan; 4Creighton University Medical Center, Phoenix, AZ
Introduction: Primary biliary cirrhosis (PBC) is a chronic autoimmune liver disease for which several therapies are under investigation. Placebo-controlled trials are commonly used to evaluate the efficacy of new treatments, but a clear understanding of the safety profile of placebo in this population is limited. This meta-analysis aimed to systematically assess the incidence and nature of adverse events associated with placebo administration in patients with PBC.
Methods: This systematic review and meta-analysis was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. A comprehensive literature search was performed to identify randomized controlled trials (RCTs) reporting adverse events in the placebo arms of studies involving patients with primary biliary cirrhosis (PBC). Articles were searched from inception to April 2025 across five electronic databases: PubMed, Google Scholar, Scopus, Embase, and Cochrane Central Register of Controlled Trials. Descriptive statistics were used to calculate pooled event rates, which were expressed as percentages of the total placebo population.
Results: A total of 10 randomized controlled trials were included in this meta-analysis, comprising 633 patients who received placebo for the treatment of primary biliary cirrhosis. The analysis focused on the incidence of adverse events reported in the placebo arms. The most commonly observed adverse event was pruritus, reported in 17.2% of patients (109 cases). Other frequent adverse events included headache (5.2%), nausea (5.1%), abdominal pain (3.6%), diarrhea (3.5%), and urinary tract infections (UTI) (3.0%). Less common adverse events were nasopharyngitis (2.2%), arthralgia (1.4%), and upper respiratory tract infections (URTI) (1.3%). Rarely reported events included constipation (0.8%), abdominal distension (0.5%), and insomnia (0.2%).
Discussion: This meta-analysis demonstrates that a placebo is generally safe and well-tolerated in patients with primary biliary cirrhosis. Most adverse events were mild, infrequent, and self-limiting.
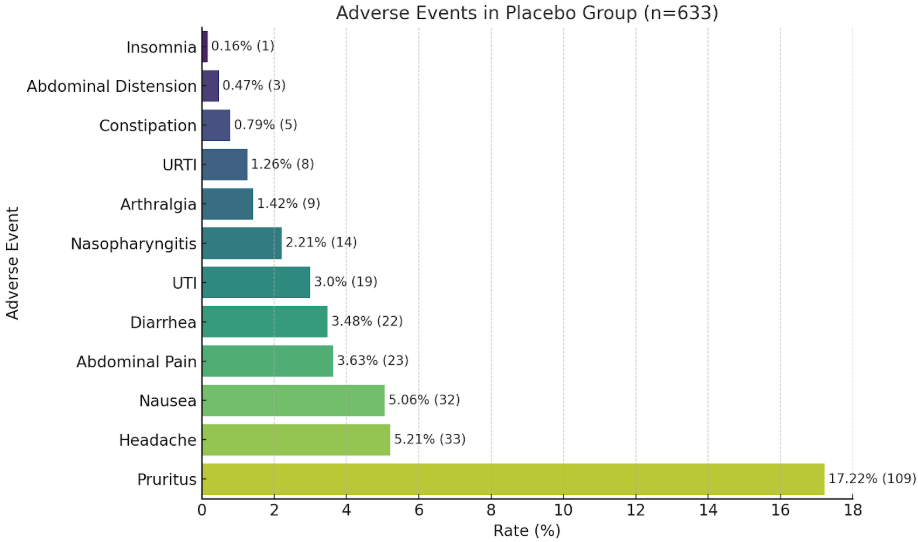
Figure: Rate of adverse events in patients receiving placebo
Disclosures:
Sampada Bhasker indicated no relevant financial relationships.
Aalam Sohal indicated no relevant financial relationships.
Vikash Karmani indicated no relevant financial relationships.
Vikash Kumar indicated no relevant financial relationships.
Divyesh Sejpal: Boston Scientific – Consultant. Olympus – Grant/Research Support.
Sampada Bhasker, MBBS1, Aalam Sohal, MD2, Vikash Karmani, MBBS3, Vikash Kumar, MD4, Divyesh Sejpal, MD, MS2. P3753 - Safety Profile of Placebo in Patients With Primary Biliary Cirrhosis: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Punjab Institute of Medical Sciences, Jalandhar, Punjab, India; 2Creighton University School of Medicine, Phoenix, AZ; 3Jinnah Sindh Medical University, Karachi, Sindh, Pakistan; 4Creighton University Medical Center, Phoenix, AZ
Introduction: Primary biliary cirrhosis (PBC) is a chronic autoimmune liver disease for which several therapies are under investigation. Placebo-controlled trials are commonly used to evaluate the efficacy of new treatments, but a clear understanding of the safety profile of placebo in this population is limited. This meta-analysis aimed to systematically assess the incidence and nature of adverse events associated with placebo administration in patients with PBC.
Methods: This systematic review and meta-analysis was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. A comprehensive literature search was performed to identify randomized controlled trials (RCTs) reporting adverse events in the placebo arms of studies involving patients with primary biliary cirrhosis (PBC). Articles were searched from inception to April 2025 across five electronic databases: PubMed, Google Scholar, Scopus, Embase, and Cochrane Central Register of Controlled Trials. Descriptive statistics were used to calculate pooled event rates, which were expressed as percentages of the total placebo population.
Results: A total of 10 randomized controlled trials were included in this meta-analysis, comprising 633 patients who received placebo for the treatment of primary biliary cirrhosis. The analysis focused on the incidence of adverse events reported in the placebo arms. The most commonly observed adverse event was pruritus, reported in 17.2% of patients (109 cases). Other frequent adverse events included headache (5.2%), nausea (5.1%), abdominal pain (3.6%), diarrhea (3.5%), and urinary tract infections (UTI) (3.0%). Less common adverse events were nasopharyngitis (2.2%), arthralgia (1.4%), and upper respiratory tract infections (URTI) (1.3%). Rarely reported events included constipation (0.8%), abdominal distension (0.5%), and insomnia (0.2%).
Discussion: This meta-analysis demonstrates that a placebo is generally safe and well-tolerated in patients with primary biliary cirrhosis. Most adverse events were mild, infrequent, and self-limiting.

Figure: Rate of adverse events in patients receiving placebo
Disclosures:
Sampada Bhasker indicated no relevant financial relationships.
Aalam Sohal indicated no relevant financial relationships.
Vikash Karmani indicated no relevant financial relationships.
Vikash Kumar indicated no relevant financial relationships.
Divyesh Sejpal: Boston Scientific – Consultant. Olympus – Grant/Research Support.
Sampada Bhasker, MBBS1, Aalam Sohal, MD2, Vikash Karmani, MBBS3, Vikash Kumar, MD4, Divyesh Sejpal, MD, MS2. P3753 - Safety Profile of Placebo in Patients With Primary Biliary Cirrhosis: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
