Monday Poster Session
Category: Liver
P3749 - Association of Gender-Affirming Hormone Therapy With Development of Elevated Liver Chemistries and Liver Disease: 9-Year Cohort Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Newsha Nikzad, MD
University of Chicago
Chicago, IL
Presenting Author(s)
Newsha Nikzad, MD1, Christina Dimopoulos-Verma, MD2, Seren M. Gedallovich, MD2, Douglas Simonetto, MD3, Alexandra T. Strauss, MD, PhD, MIE4, Sonali Paul, MD, MS, FACG1, Nikki Duong, MD2
1University of Chicago, Chicago, IL; 2Stanford University, Stanford, CA; 3Mayo Clinic, Rochester, MN; 4Johns Hopkins University, Baltimore, MD
Introduction: Transgender and gender diverse (TGD) individuals in the United States are disproportionately affected by risk factors for liver disease, such as high rates of obesity, diabetes, alcohol use disorder, and viral hepatitis. Gender affirming hormone therapy (GAHT), such as estrogen and testosterone, can be fundamental care for some TGD individuals who experience gender dysphoria. However, evidence is limited regarding the association between GAHT and liver-related conditions.
Methods: In this retrospective study, we used a national electronic health record and claims database (Atropos Health) to describe the incidence of liver-related outcomes in adults receiving GAHT from 2015-2024. Adult TGD patients with ICD-10 diagnosis codes for gender identity disorder/gender dysphoria who had received either estrogen or testosterone based therapies were included. Those with a prior history of liver disease, hepatocellular carcinoma (HCC), alcohol use disorder, osteoporosis, endocrine disorders, or any abnormal hormone levels were excluded.
Results: Of the 8,673 individuals (mean age 30.8 years) who started estrogen-based GAHT, 2,654 (31%) had AST/ALT measurements recorded after one year; 93% had normal AST/ALT levels after one year of therapy. Of the 9,570 individuals (mean age 26.4 years) who initiated testosterone-based GAHT, 2,610 (27%) had AST/ALT measurements recorded; 94% had normal AST/ALT levels after one year of therapy. In both groups, ~2% of patients had a new diagnosis of steatotic liver disease. Less than 1% of individuals in both groups had an incident diagnosis of Budd-Chiari syndrome, cirrhosis, or HCC. Additionally, there were low incidences of venous thromboembolism (1.08%), hyperlipidemia (3.40%), or hypertension (1.91%) after one year of estrogen-based GAHT. Similarly, few patients were newly diagnosed with venous thromboembolism (0.68%), hyperlipidemia (3.49%), or hypertension (2.13%) after one year of testosterone-based GAHT.
Discussion: This is the largest real-world study to date assessing GAHT and liver safety, including over 8,500 estrogen and 9,500 testosterone users over 9 years. GAHT was associated with low rates of liver enzyme abnormalities, new liver disease, and metabolic complications after one year, suggesting hepatic safety. A multidisciplinary approach remains essential, and prospective studies are needed to explore formulation-specific risks. This study addresses a key gap in existing knowledge.
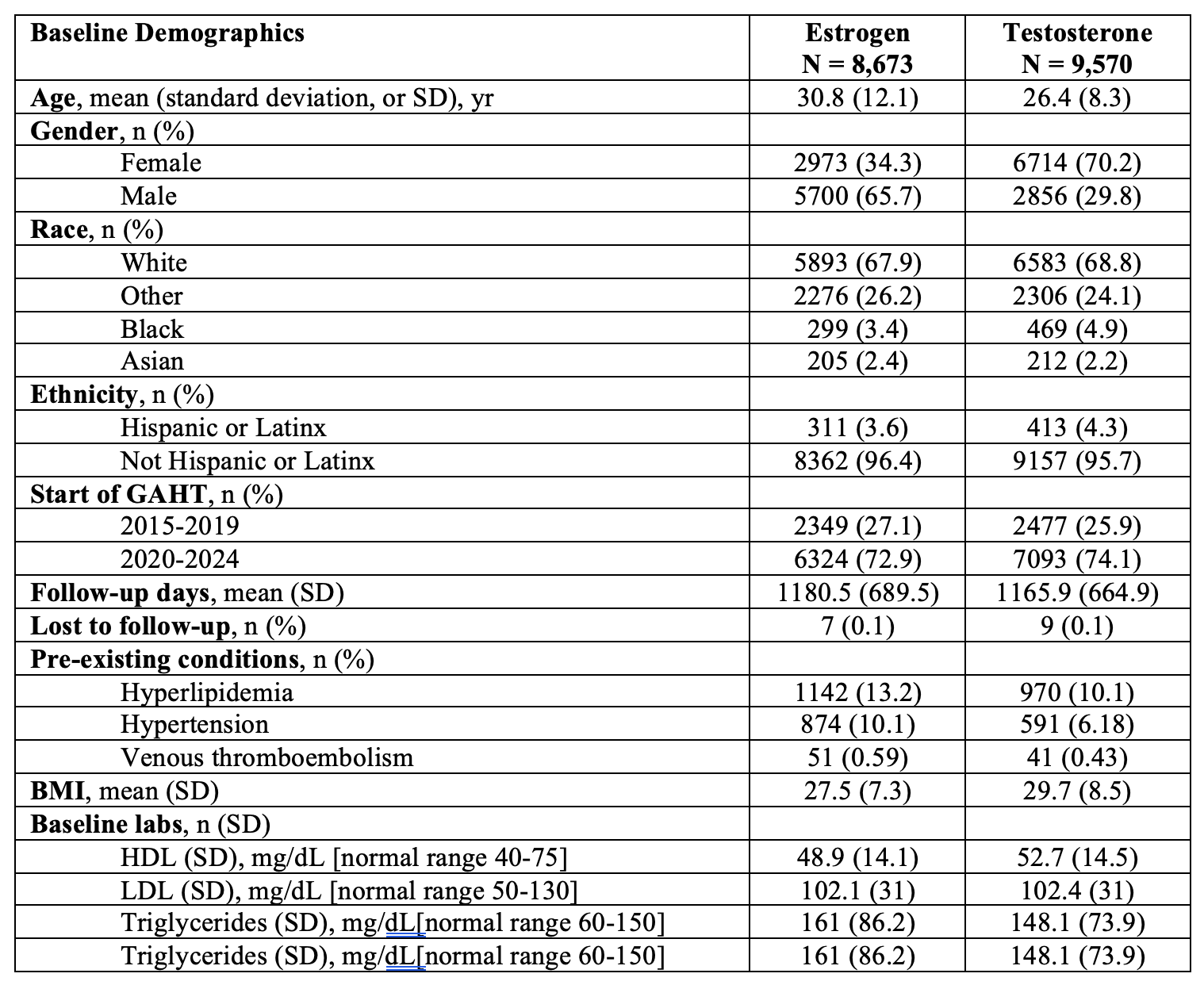
Figure: Table 1: Cohort characteristics based on exposure to therapeutic estrogen or testosterone
Disclosures:
Newsha Nikzad indicated no relevant financial relationships.
Christina Dimopoulos-Verma indicated no relevant financial relationships.
Seren Gedallovich indicated no relevant financial relationships.
Douglas Simonetto indicated no relevant financial relationships.
Alexandra Strauss indicated no relevant financial relationships.
Sonali Paul: Eli Lilly – Stock-publicly held company(excluding mutual/index funds).
Nikki Duong indicated no relevant financial relationships.
Newsha Nikzad, MD1, Christina Dimopoulos-Verma, MD2, Seren M. Gedallovich, MD2, Douglas Simonetto, MD3, Alexandra T. Strauss, MD, PhD, MIE4, Sonali Paul, MD, MS, FACG1, Nikki Duong, MD2. P3749 - Association of Gender-Affirming Hormone Therapy With Development of Elevated Liver Chemistries and Liver Disease: 9-Year Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Chicago, Chicago, IL; 2Stanford University, Stanford, CA; 3Mayo Clinic, Rochester, MN; 4Johns Hopkins University, Baltimore, MD
Introduction: Transgender and gender diverse (TGD) individuals in the United States are disproportionately affected by risk factors for liver disease, such as high rates of obesity, diabetes, alcohol use disorder, and viral hepatitis. Gender affirming hormone therapy (GAHT), such as estrogen and testosterone, can be fundamental care for some TGD individuals who experience gender dysphoria. However, evidence is limited regarding the association between GAHT and liver-related conditions.
Methods: In this retrospective study, we used a national electronic health record and claims database (Atropos Health) to describe the incidence of liver-related outcomes in adults receiving GAHT from 2015-2024. Adult TGD patients with ICD-10 diagnosis codes for gender identity disorder/gender dysphoria who had received either estrogen or testosterone based therapies were included. Those with a prior history of liver disease, hepatocellular carcinoma (HCC), alcohol use disorder, osteoporosis, endocrine disorders, or any abnormal hormone levels were excluded.
Results: Of the 8,673 individuals (mean age 30.8 years) who started estrogen-based GAHT, 2,654 (31%) had AST/ALT measurements recorded after one year; 93% had normal AST/ALT levels after one year of therapy. Of the 9,570 individuals (mean age 26.4 years) who initiated testosterone-based GAHT, 2,610 (27%) had AST/ALT measurements recorded; 94% had normal AST/ALT levels after one year of therapy. In both groups, ~2% of patients had a new diagnosis of steatotic liver disease. Less than 1% of individuals in both groups had an incident diagnosis of Budd-Chiari syndrome, cirrhosis, or HCC. Additionally, there were low incidences of venous thromboembolism (1.08%), hyperlipidemia (3.40%), or hypertension (1.91%) after one year of estrogen-based GAHT. Similarly, few patients were newly diagnosed with venous thromboembolism (0.68%), hyperlipidemia (3.49%), or hypertension (2.13%) after one year of testosterone-based GAHT.
Discussion: This is the largest real-world study to date assessing GAHT and liver safety, including over 8,500 estrogen and 9,500 testosterone users over 9 years. GAHT was associated with low rates of liver enzyme abnormalities, new liver disease, and metabolic complications after one year, suggesting hepatic safety. A multidisciplinary approach remains essential, and prospective studies are needed to explore formulation-specific risks. This study addresses a key gap in existing knowledge.

Figure: Table 1: Cohort characteristics based on exposure to therapeutic estrogen or testosterone
Disclosures:
Newsha Nikzad indicated no relevant financial relationships.
Christina Dimopoulos-Verma indicated no relevant financial relationships.
Seren Gedallovich indicated no relevant financial relationships.
Douglas Simonetto indicated no relevant financial relationships.
Alexandra Strauss indicated no relevant financial relationships.
Sonali Paul: Eli Lilly – Stock-publicly held company(excluding mutual/index funds).
Nikki Duong indicated no relevant financial relationships.
Newsha Nikzad, MD1, Christina Dimopoulos-Verma, MD2, Seren M. Gedallovich, MD2, Douglas Simonetto, MD3, Alexandra T. Strauss, MD, PhD, MIE4, Sonali Paul, MD, MS, FACG1, Nikki Duong, MD2. P3749 - Association of Gender-Affirming Hormone Therapy With Development of Elevated Liver Chemistries and Liver Disease: 9-Year Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

