Tuesday Poster Session
Category: Colon
P4611 - Use of Tumor-Informed Circulating Tumor DNA and EUS for the Detection of Rectal Cancer Recurrence After Total Neoadjuvant Therapy
- DF
Douglas Faigel, MD (he/him/his)
Mayo Clinic
Scottsdale, AZ
Presenting Author(s)
Award: ACG Presidential Poster Award
Douglas Faigel, MD1, Tonia Young-Fadok, MD1, William Rule, MD1, Christina Wu, MBBS2
1Mayo Clinic, Scottsdale, AZ; 2Mayo Clinic, Phoenix, AZ
Introduction:
Neoadjuvant protocols with curative intent, termed Total Neoadjuvant Therapy (TNT), have achieved high complete response rates allowing many patients to avoid surgery, and reserving resection for those with persistent or recurrent disease. Close follow-up is mandatory and relies on cross sectional imaging, tumor markers, sigmoidoscopy and EUS. Tumor informed circulating tumor DNA (ctDNA) testing uses genetic sequencing of the patient’s tumor to develop PCR probes to assess for ctDNA in the blood and may have a role in post-TNT surveillance
Case Description/
Methods:
44 yo woman was diagnosed with rectal cancer staged T3bN0Mx by MRI in February 2023. She received chemoradiation with capecitabine and 50.4 Gy in 25 fractions, followed by 5-Fluorouracil and oxaliplatin (FOLFOX) x 8 cycles achieving a complete response by imaging, sigmoidoscopy and EUS. Surveillance included ctDNA (Signatera, Natera, Austin TX), MRI and EUS with endoscopic biopsies of the scar. The ctDNA was negative from November 2023 until August of 2024 when it was weakly positive (0.04 MTM/ml). MRI and EUS in September and December 2024 showed a stable scar and mucosal biopsies were negative. CEA was normal. February 2025 ctDNA had risen to 0.26 MTM/ml. In March 2025 EUS showed an increase in the size of the scar from 20 mm wide by 7 mm thick in September 2024 (Figure 1) to 25 mm wide by 10 mm thick (Figure 2). Mucosal biopsies were again negative. EUS-guided FNB using a 25 gauge needle was positive for adenocarcinoma. PET MRI 1 week later showed an enlarged region of diffusion restriction in the scar with focal radiotracer update but no metastases. Patient underwent a robotic-assisted low anterior resection with final pathology showing residual adenocarcinoma with negative margins and 0/13 nodes positive.
Fig 1. EUS Sept 2024 showing thickened irregular scar
Fig 2. EUS March 2025 showing interval enlargement
Discussion:
TNT achieves clinical complete response in rectal cancer of up to 80% at 1 year. This has led to new treatment algorithms reserving surgical resections for those with locally persistent or recurrent disease, thus avoiding the potential need for a permanent colostomy. Close follow-up is mandatory. ctDNA has sensitivity and specificity for CRC recurrence of up to 70% and 90%, respectively, and may be useful to detect recurrences before they are clinically apparent. A positive ctDNA should prompt heightened surveillance with low threshold for obtaining deep biopsies of even stable appearing post treatment scars.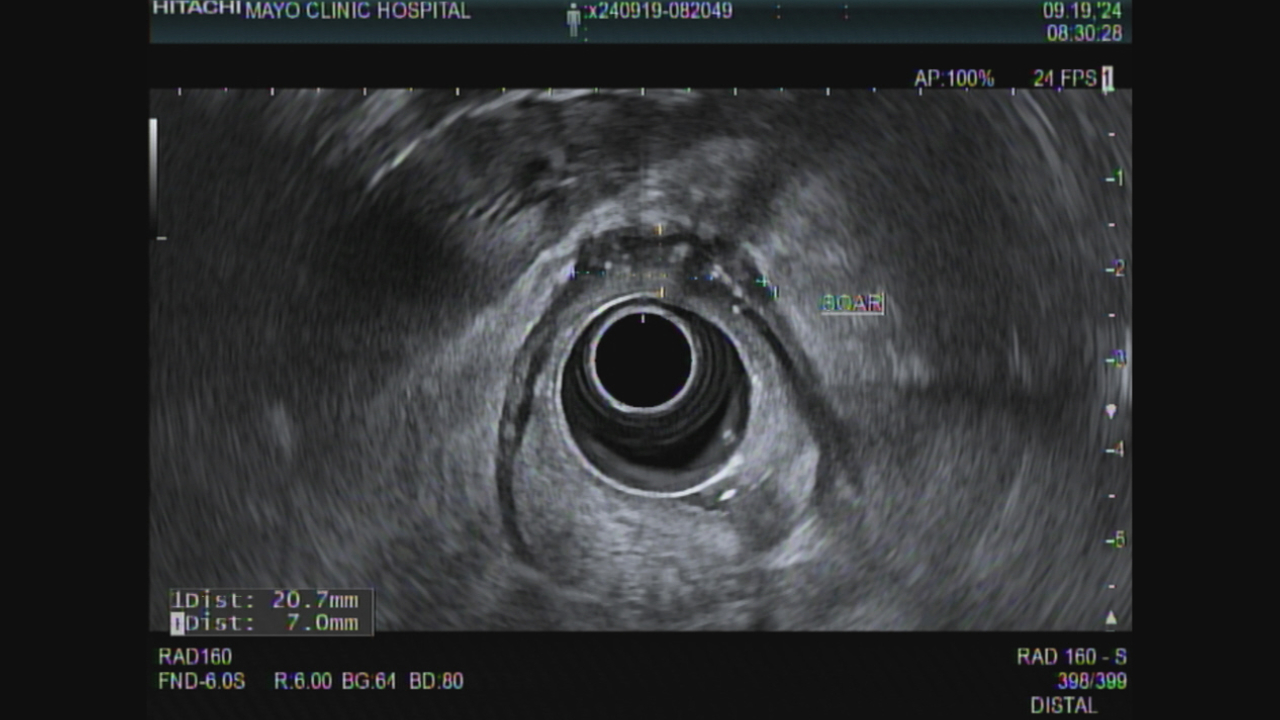
Figure: Figure 1. Rectal EUS Sept 2024 showing thickened irregular scar 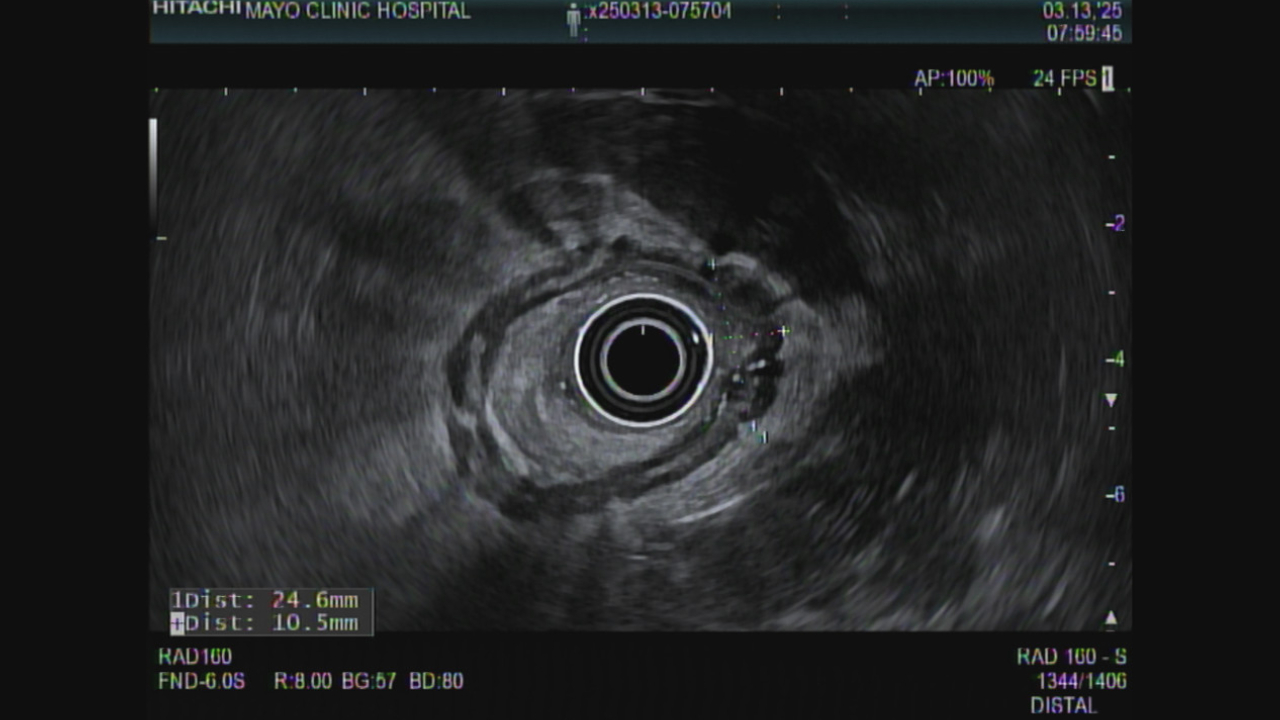
Figure: Figure 2. Rectal EUS March 2025 showing interval enlargement of the scar
Disclosures:
Douglas Faigel indicated no relevant financial relationships.
Tonia Young-Fadok indicated no relevant financial relationships.
William Rule indicated no relevant financial relationships.
Christina Wu: DoMore Diagnostics – Advisor or Review Panel Member. Exelixis – Advisor or Review Panel Member. GlaxkoSmithKline – Advisor or Review Panel Member. Merck – Advisor or Review Panel Member. Natera – Advisor or Review Panel Member. Pfizer – Grant/Research Support. Seagen – Grant/Research Support.
Douglas Faigel, MD1, Tonia Young-Fadok, MD1, William Rule, MD1, Christina Wu, MBBS2. P4611 - Use of Tumor-Informed Circulating Tumor DNA and EUS for the Detection of Rectal Cancer Recurrence After Total Neoadjuvant Therapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.


