Tuesday Poster Session
Category: Esophagus
P4959 - Immune Checkpoint Inhibitor-Induced Esophagitis Mimicking Reflux and Infectious Etiologies: A Rare but Emerging Gastrointestinal Immune-Related Adverse Effect
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Corey Shamley, MD
University of Alabama at Birmingham Heersink School of Medicine
Birmingham, AL
Presenting Author(s)
Malcolm B. Chapman, MD, MBA1, Corey Shamley, MD1, Behiye Goksel, MD1, Goo Lee, MD1, Amia Bolin, CRNP1, James Callaway, MD2, Frederick Weber, MD1
1University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 2Basil I. Hirschowitz Endoscopic Center of Excellence, The University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL
Introduction: Immune checkpoint inhibitor (ICI) use continues to increase as they have become a cornerstone in the treatment of numerous cancers. ICI-induced esophagitis is a rare but significant immune-related adverse event (irAE) associated with the use of ICIs such as anti-PD-1, anti-PD-L1, and anti-CTLA-4 therapies.
Case Description/
Methods: We present a case of a 79-year-old female with relevant history of high-grade, metastatic neuroendocrine bladder cancer on pembrolizumab who presented to the hospital 10 months after ICI initiation with odynophagia and dysphagia of 2-month duration. Their symptoms had been previously evaluated in an emergency department and in two urgent care visits. Initially prescribed viscous lidocaine and prednisone with brief symptomatic improvement, they experienced symptom recurrence after completion of corticosteroids. Then, their symptoms persisted despite an extended course of fluconazole prescribed to empirically treat suspected esophageal candidiasis. Four weeks after hospital discharge, the patient underwent an outpatient esophagogastroduodenoscopy (EGD) that found many linear ulcerations ranging from 2-10 cm throughout the entire esophagus (Figure 1A). Subsequent biopsies revealed lymphocytic inflammation of squamous epithelium with apoptotic bodies consistent with ICI-induced esophagitis (Figure 1B). Pembrolizumab was held for 4 weeks while they received an oral budesonide taper and esomeprazole. Eight weeks after initiation of glucocorticoid and proton-pump inhibitor, the patient returned for outpatient follow-up and endorsed complete resolution of odynophagia and dysphagia. An EGD was repeated, demonstrating resolution of linear esophageal ulcerations, normal mucosa of the upper-third esophagus, textured mucosal changes of the middle-third of esophagus, and non-obstructing stenosis at the same level (Figure 2A). Biopsies from the post-treatment EGD demonstrated improvement in the number of dyskeratotic keratinocytes and dramatic reduction in intraepithelial lymphocytes (Figure 2B).
Discussion: Although rare, it is increasingly important that physicians be able to recognize and treat ICI-induced esophagitis. Clinically, the presentation of this irAE may mimic other, more common, forms of esophagitis. However, it has a distinct histopathological appearance on biopsy that necessitates EGD to confirm the diagnosis.
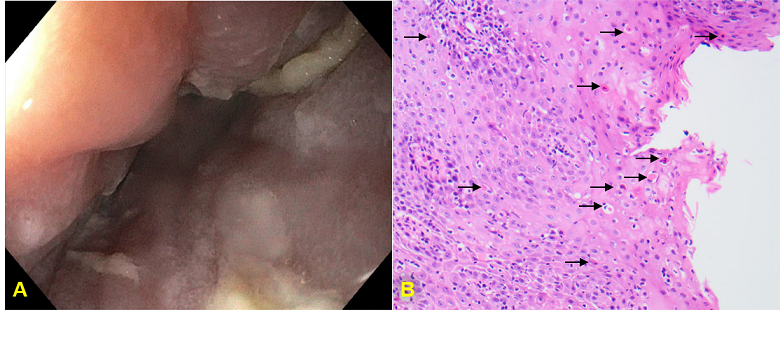
Figure: A) Initial EGD finding numerous 2-10 cm linear ulcerations at middle third of esophagus. B) Esophageal squamous mucosa showing numerous dyskeratotic keratinocytes (black arrow) and markedly increased intraepithelial lymphocytes.
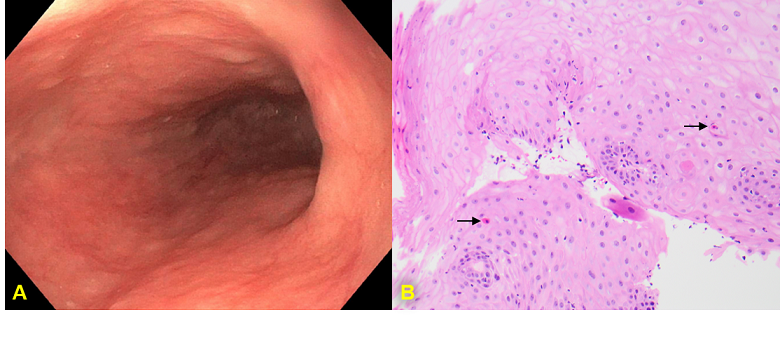
Figure: A) Follow-up EGD finding scattered mild mucosal changes characterized by altered texture and non-obstructing stenosis at middle third of esophagus. B) Post-treatment biopsy demonstrates only rare dyskeratotic keratinocytes (black arrow) and significantly decreased intraepithelial lymphocytes.
Disclosures:
Malcolm Chapman indicated no relevant financial relationships.
Corey Shamley indicated no relevant financial relationships.
Behiye Goksel indicated no relevant financial relationships.
Goo Lee indicated no relevant financial relationships.
Amia Bolin: Enterra Medical – Advisor or Review Panel Member. Sanofi – Advisory Committee/Board Member.
James Callaway indicated no relevant financial relationships.
Frederick Weber indicated no relevant financial relationships.
Malcolm B. Chapman, MD, MBA1, Corey Shamley, MD1, Behiye Goksel, MD1, Goo Lee, MD1, Amia Bolin, CRNP1, James Callaway, MD2, Frederick Weber, MD1. P4959 - Immune Checkpoint Inhibitor-Induced Esophagitis Mimicking Reflux and Infectious Etiologies: A Rare but Emerging Gastrointestinal Immune-Related Adverse Effect, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 2Basil I. Hirschowitz Endoscopic Center of Excellence, The University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL
Introduction: Immune checkpoint inhibitor (ICI) use continues to increase as they have become a cornerstone in the treatment of numerous cancers. ICI-induced esophagitis is a rare but significant immune-related adverse event (irAE) associated with the use of ICIs such as anti-PD-1, anti-PD-L1, and anti-CTLA-4 therapies.
Case Description/
Methods: We present a case of a 79-year-old female with relevant history of high-grade, metastatic neuroendocrine bladder cancer on pembrolizumab who presented to the hospital 10 months after ICI initiation with odynophagia and dysphagia of 2-month duration. Their symptoms had been previously evaluated in an emergency department and in two urgent care visits. Initially prescribed viscous lidocaine and prednisone with brief symptomatic improvement, they experienced symptom recurrence after completion of corticosteroids. Then, their symptoms persisted despite an extended course of fluconazole prescribed to empirically treat suspected esophageal candidiasis. Four weeks after hospital discharge, the patient underwent an outpatient esophagogastroduodenoscopy (EGD) that found many linear ulcerations ranging from 2-10 cm throughout the entire esophagus (Figure 1A). Subsequent biopsies revealed lymphocytic inflammation of squamous epithelium with apoptotic bodies consistent with ICI-induced esophagitis (Figure 1B). Pembrolizumab was held for 4 weeks while they received an oral budesonide taper and esomeprazole. Eight weeks after initiation of glucocorticoid and proton-pump inhibitor, the patient returned for outpatient follow-up and endorsed complete resolution of odynophagia and dysphagia. An EGD was repeated, demonstrating resolution of linear esophageal ulcerations, normal mucosa of the upper-third esophagus, textured mucosal changes of the middle-third of esophagus, and non-obstructing stenosis at the same level (Figure 2A). Biopsies from the post-treatment EGD demonstrated improvement in the number of dyskeratotic keratinocytes and dramatic reduction in intraepithelial lymphocytes (Figure 2B).
Discussion: Although rare, it is increasingly important that physicians be able to recognize and treat ICI-induced esophagitis. Clinically, the presentation of this irAE may mimic other, more common, forms of esophagitis. However, it has a distinct histopathological appearance on biopsy that necessitates EGD to confirm the diagnosis.

Figure: A) Initial EGD finding numerous 2-10 cm linear ulcerations at middle third of esophagus. B) Esophageal squamous mucosa showing numerous dyskeratotic keratinocytes (black arrow) and markedly increased intraepithelial lymphocytes.

Figure: A) Follow-up EGD finding scattered mild mucosal changes characterized by altered texture and non-obstructing stenosis at middle third of esophagus. B) Post-treatment biopsy demonstrates only rare dyskeratotic keratinocytes (black arrow) and significantly decreased intraepithelial lymphocytes.
Disclosures:
Malcolm Chapman indicated no relevant financial relationships.
Corey Shamley indicated no relevant financial relationships.
Behiye Goksel indicated no relevant financial relationships.
Goo Lee indicated no relevant financial relationships.
Amia Bolin: Enterra Medical – Advisor or Review Panel Member. Sanofi – Advisory Committee/Board Member.
James Callaway indicated no relevant financial relationships.
Frederick Weber indicated no relevant financial relationships.
Malcolm B. Chapman, MD, MBA1, Corey Shamley, MD1, Behiye Goksel, MD1, Goo Lee, MD1, Amia Bolin, CRNP1, James Callaway, MD2, Frederick Weber, MD1. P4959 - Immune Checkpoint Inhibitor-Induced Esophagitis Mimicking Reflux and Infectious Etiologies: A Rare but Emerging Gastrointestinal Immune-Related Adverse Effect, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
