Tuesday Poster Session
Category: IBD
P5346 - Assessing the Risk of Inflammatory Bowel Disease Following Ocrelizumab Use in Multiple Sclerosis Patients: A Retrospective Analysis Utilizing a Large National Database
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- MM
Mouhand Mohamed, MBBS, MS
Mayo Clinic
Rochester, MN
Presenting Author(s)
Mouhand Mohamed, MBBS, MS1, Azizullah Beran, MD2, Osama Hamid, MD3, Darrell Pardi, MD, MS, FACG1
1Mayo Clinic, Rochester, MN; 2Indiana University School of Medicine, Indianapolis, IN; 3University of Texas Southwestern Medical Center, Dallas, TX
Introduction: Ocrelizumab is an anti-CD20 monoclonal antibody approved for the treatment of multiple sclerosis (MS), including primary progressive MS. Postmarketing surveillance identified cases of immune-mediated colitis in Ocrelizumab users, some requiring hospitalization or even surgical intervention. Given this potential safety signal and the limited published data, we aimed to evaluate the real-world risk of inflammatory bowel disease (IBD) and colitis in MS patients treated with Ocrelizumab.
Methods: We utilized the TriNetX database, comprising > 100 million patient records. Using ICD-10 codes, adult MS patients (≥18 years) were identified based on > 3 recorded MS diagnoses and receipt of a disease-modifying therapy. MS patients treated with Ocrelizumab were compared to MS patients receiving other disease-modifying therapies. Propensity score matching (PSM) was performed for Age, sex, race, tobacco use, diabetes mellitus, CKD, and hypertension. Outcomes assessed included IBD, noninfectious and infectious colitis, colectomy, and use of vedolizumab or anti-tumor necrosis factor (TNF) agents. Hazard ratios (HR) with 95% confidence intervals (CI) were generated for statistical comparison. P value cutoff < 0.5 was considered statistically significant.
Results: After PSM, 22,513 patients were included in each group. The median follow-up time was 1,332 days in Ocrelizumab users compared to 1,609 days in controls. There was no statistically significant increase in IBD risk among Ocrelizumab users compared to controls (1 vs. 1.1%; HR 1.03, 95% CI 0.86–1.24). Similarly, no significant differences were observed in colectomy (HR 0.72, 95% CI 0.45–1.15), use of vedolizumab or anti-TNF (HR 0.88, 95% CI 0.51–1.51), noninfectious colitis or enteritis (HR 1.02, 95% CI 0.93–1.12) or infectious colitis or enteritis (HR, 0.20; 95% CI, 0.02–1.72).
Discussion: The findings from this large representative cohort did not reveal an association between Ocrelizumab use and increased risk of IBD or colitis. Based on a risk-benefit assessment, it may be reasonable to consider continuing Ocrelizumab therapy in appropriate patients, particularly given its unique approval for primary progressive multiple sclerosis. Clinical monitoring for gastrointestinal symptoms remains important. Limitations of this study include the retrospective design, reliance on ICD codes, and inability to rule out residual confounding. Further research is needed to confirm these findings.
Disclosure: Supervised AI assisted with generating the tables.
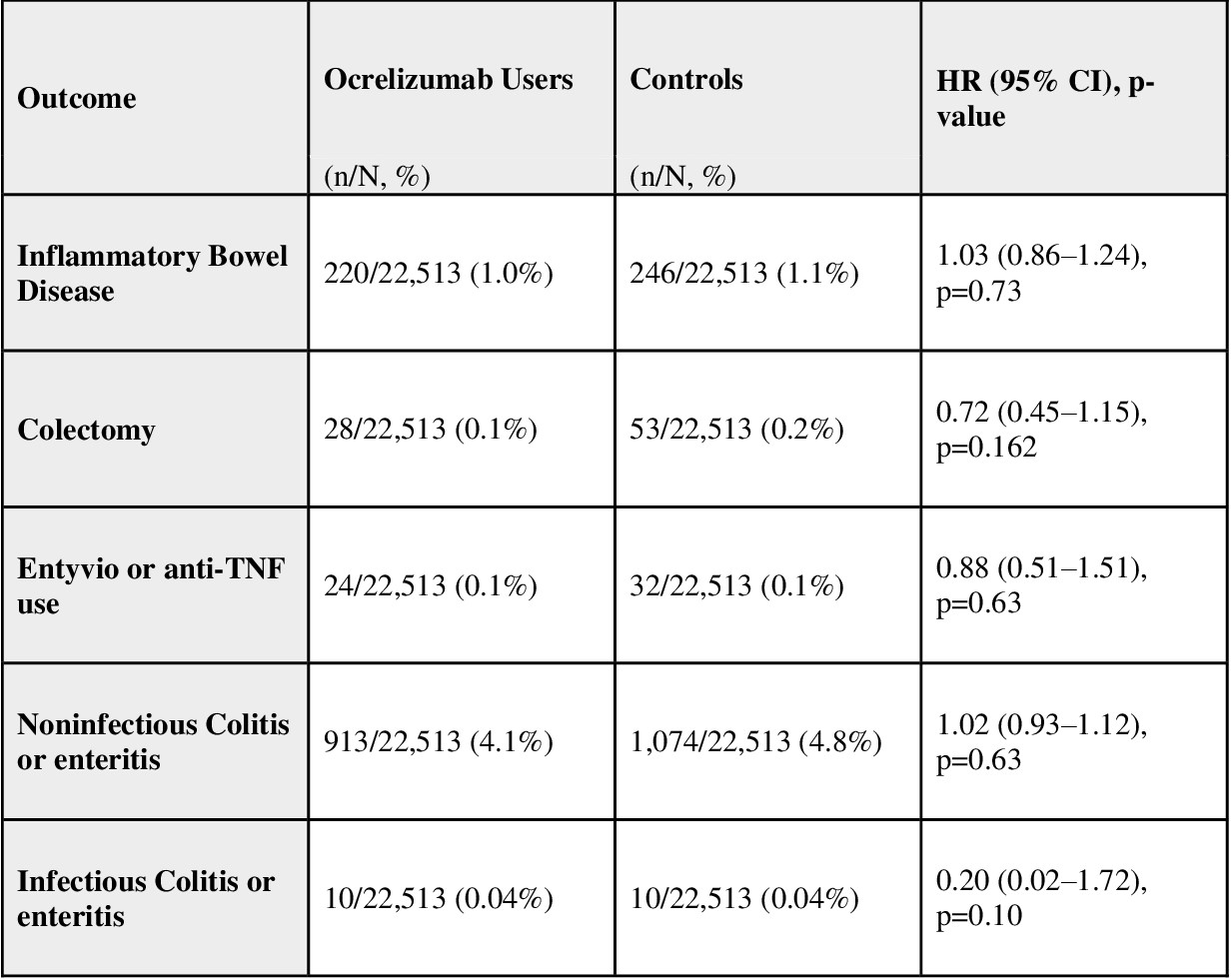
Figure: Table 1: Baseline Characteristics After Propensity Score Matching
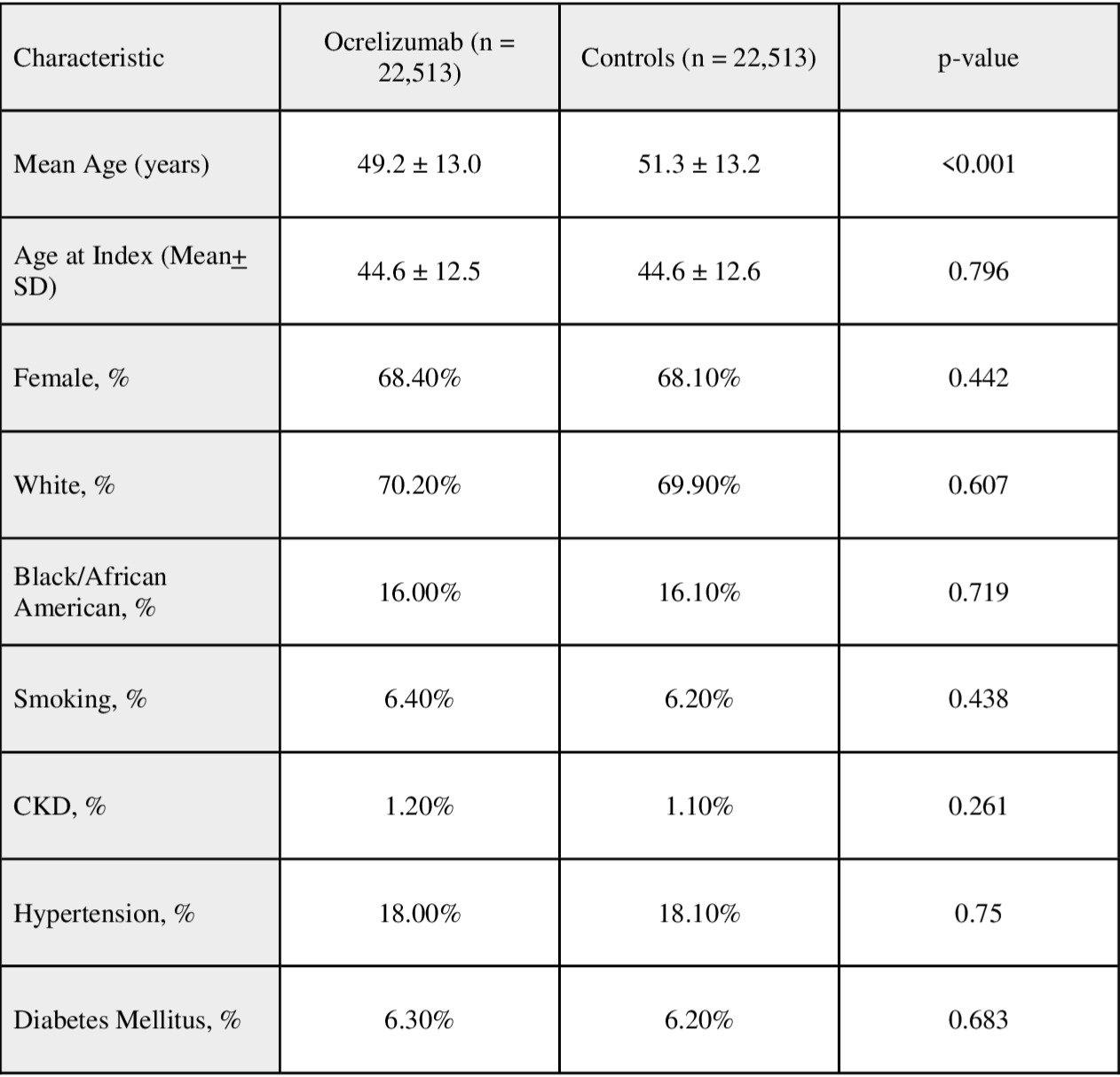
Figure: Table 2. Summary of Study Outcomes
Disclosures:
Mouhand Mohamed indicated no relevant financial relationships.
Azizullah Beran indicated no relevant financial relationships.
Osama Hamid indicated no relevant financial relationships.
Darrell Pardi: Applied Molecular Transport – Consultant. ExeGI Pharma LC – Grant/Research Support. Janssen – Advisory Committee/Board Member. Lilly Medical – Consultant. Pfizer – Consultant. Rise Therapeutics – Grant/Research Support. Takeda – Consultant. Vedanta Bio Sciences INC – Grant/Research Support.
Mouhand Mohamed, MBBS, MS1, Azizullah Beran, MD2, Osama Hamid, MD3, Darrell Pardi, MD, MS, FACG1. P5346 - Assessing the Risk of Inflammatory Bowel Disease Following Ocrelizumab Use in Multiple Sclerosis Patients: A Retrospective Analysis Utilizing a Large National Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2Indiana University School of Medicine, Indianapolis, IN; 3University of Texas Southwestern Medical Center, Dallas, TX
Introduction: Ocrelizumab is an anti-CD20 monoclonal antibody approved for the treatment of multiple sclerosis (MS), including primary progressive MS. Postmarketing surveillance identified cases of immune-mediated colitis in Ocrelizumab users, some requiring hospitalization or even surgical intervention. Given this potential safety signal and the limited published data, we aimed to evaluate the real-world risk of inflammatory bowel disease (IBD) and colitis in MS patients treated with Ocrelizumab.
Methods: We utilized the TriNetX database, comprising > 100 million patient records. Using ICD-10 codes, adult MS patients (≥18 years) were identified based on > 3 recorded MS diagnoses and receipt of a disease-modifying therapy. MS patients treated with Ocrelizumab were compared to MS patients receiving other disease-modifying therapies. Propensity score matching (PSM) was performed for Age, sex, race, tobacco use, diabetes mellitus, CKD, and hypertension. Outcomes assessed included IBD, noninfectious and infectious colitis, colectomy, and use of vedolizumab or anti-tumor necrosis factor (TNF) agents. Hazard ratios (HR) with 95% confidence intervals (CI) were generated for statistical comparison. P value cutoff < 0.5 was considered statistically significant.
Results: After PSM, 22,513 patients were included in each group. The median follow-up time was 1,332 days in Ocrelizumab users compared to 1,609 days in controls. There was no statistically significant increase in IBD risk among Ocrelizumab users compared to controls (1 vs. 1.1%; HR 1.03, 95% CI 0.86–1.24). Similarly, no significant differences were observed in colectomy (HR 0.72, 95% CI 0.45–1.15), use of vedolizumab or anti-TNF (HR 0.88, 95% CI 0.51–1.51), noninfectious colitis or enteritis (HR 1.02, 95% CI 0.93–1.12) or infectious colitis or enteritis (HR, 0.20; 95% CI, 0.02–1.72).
Discussion: The findings from this large representative cohort did not reveal an association between Ocrelizumab use and increased risk of IBD or colitis. Based on a risk-benefit assessment, it may be reasonable to consider continuing Ocrelizumab therapy in appropriate patients, particularly given its unique approval for primary progressive multiple sclerosis. Clinical monitoring for gastrointestinal symptoms remains important. Limitations of this study include the retrospective design, reliance on ICD codes, and inability to rule out residual confounding. Further research is needed to confirm these findings.
Disclosure: Supervised AI assisted with generating the tables.

Figure: Table 1: Baseline Characteristics After Propensity Score Matching

Figure: Table 2. Summary of Study Outcomes
Disclosures:
Mouhand Mohamed indicated no relevant financial relationships.
Azizullah Beran indicated no relevant financial relationships.
Osama Hamid indicated no relevant financial relationships.
Darrell Pardi: Applied Molecular Transport – Consultant. ExeGI Pharma LC – Grant/Research Support. Janssen – Advisory Committee/Board Member. Lilly Medical – Consultant. Pfizer – Consultant. Rise Therapeutics – Grant/Research Support. Takeda – Consultant. Vedanta Bio Sciences INC – Grant/Research Support.
Mouhand Mohamed, MBBS, MS1, Azizullah Beran, MD2, Osama Hamid, MD3, Darrell Pardi, MD, MS, FACG1. P5346 - Assessing the Risk of Inflammatory Bowel Disease Following Ocrelizumab Use in Multiple Sclerosis Patients: A Retrospective Analysis Utilizing a Large National Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
