Tuesday Poster Session
Category: IBD
P5341 - A Multicenter Study Identifying the Risk of Psoriasis in Patients with Inflammatory Bowel Disease Treated with TNF-α Inhibitors
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- OA
Omar Al Ta'ani, MD
Department of Internal Medicine, Allegheny Health Network, Pittsburgh, Pennsylvania, USA
Pittsburgh, PA
Presenting Author(s)
Omar Al Ta’ani, MD1, Jana G. Hashash, MD, MSc, FACG2, Yahya Alhalalmeh, MD3, Zain Al Ta'ani, MD4, Francis A.. Farraye, MD, MSc, MACG2, Saqr Alsakarneh, MD, MSc5
1Department of Internal Medicine, Allegheny Health Network, Pittsburgh, Pennsylvania, USA, Pittsburgh, PA; 2Mayo Clinic, Jacksonville, FL; 3New York Medical College - Saint Michael's Medical Center, Newark, NJ; 4University of Jordan, Amman, 'Amman, Jordan; 5Mayo Clinic, Rochester, MN
Introduction: Tumor necrosis factor-alpha (TNF-α) inhibitors are widely used in the treatment of inflammatory bowel disease (IBD), with proven efficacy in achieving and maintaining remission. However, these agents have been implicated in paradoxical immune-mediated adverse effects, including psoriasis. This study aimed to quantify the risk of incident psoriasis and psoriatic arthritis in patients with IBD receiving TNF-α inhibitor
Methods: We conducted a retrospective cohort study using the TriNetX research network, identifying adult patients with IBD who initiated treatment with either TNF-α inhibitors or non–TNF-α-based treatments. Propensity score matching (1:1) was applied to balance baseline characteristics, including age, sex, comorbidities, and IBD subtype. The primary outcome was a new diagnosis of psoriasis or psoriatic arthritis within five years of treatment initiation. Adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) were estimated using Cox proportional hazards models
Results: A total of 11,663 IBD patients treated with TNF-α inhibitors were compared to 53,977 patients receiving non–TNF-α therapies. After matching, 11,076 patients remained in each group with well-balanced characteristics (Table 1). TNF-α inhibitor use was associated with a significantly increased risk of psoriasis or psoriatic arthritis (aHR 1.63; 95% CI, 1.35–1.98; p < 0.001).
Subgroup analysis revealed significantly higher risk among females (aHR 1.90; 95% CI, 1.45–2.48; p < 0.001), while no significant association was seen in males (aHR 1.16; 95% CI, 0.85–1.58; p = 0.354). The greatest risk was observed in patients aged >60 years (aHR 2.56; 95% CI, 1.58–4.15; p < 0.001). Among individual agents, infliximab (aHR 1.53; 95% CI, 1.16–2.03; p = 0.003) and adalimumab (aHR 1.45; 95% CI, 1.09–1.92; p = 0.010) were both associated with elevated risk.
Upon stratification, both psoriasis (aHR 1.77; 95% CI, 1.46–2.14; p < 0.001) and psoriatic arthritis (aHR 1.54; 95% CI, 1.01–2.36; p = 0.048) showed significantly increased incidence in the TNF-α group, as shown in Figure 1
Discussion: In this study, TNF-α inhibitor therapy was associated with a significantly increased risk of new-onset psoriasis and psoriatic arthritis in patients with IBD. These findings underscore the importance of surveillance for paradoxical inflammatory manifestations and support individualized treatment decision-making in IBD management. Further studies are needed to better understand the mechanisms and inform strategies to minimize risk
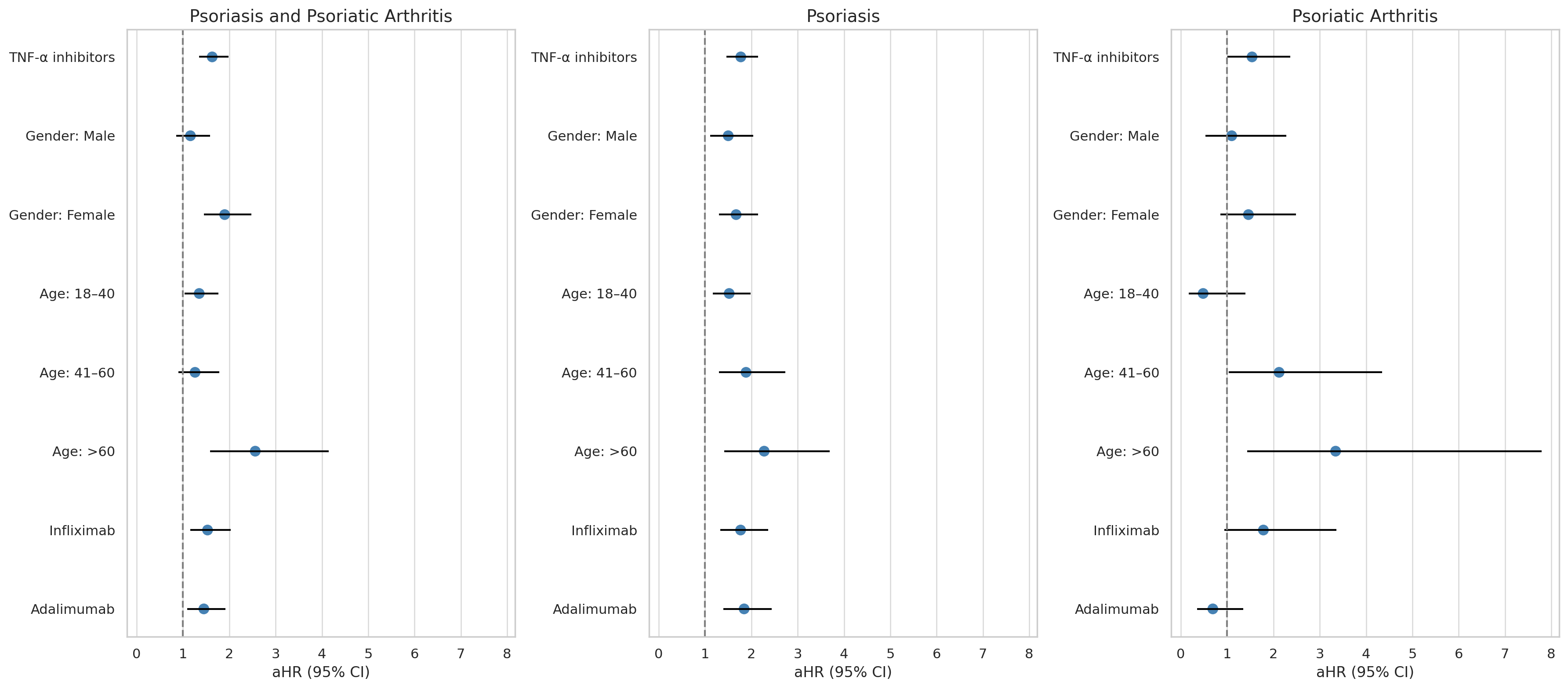
Figure: Table 1. Baseline characteristics for patients who have IBD with TNFi medication or other IBD medication before and after propensity score
matching.
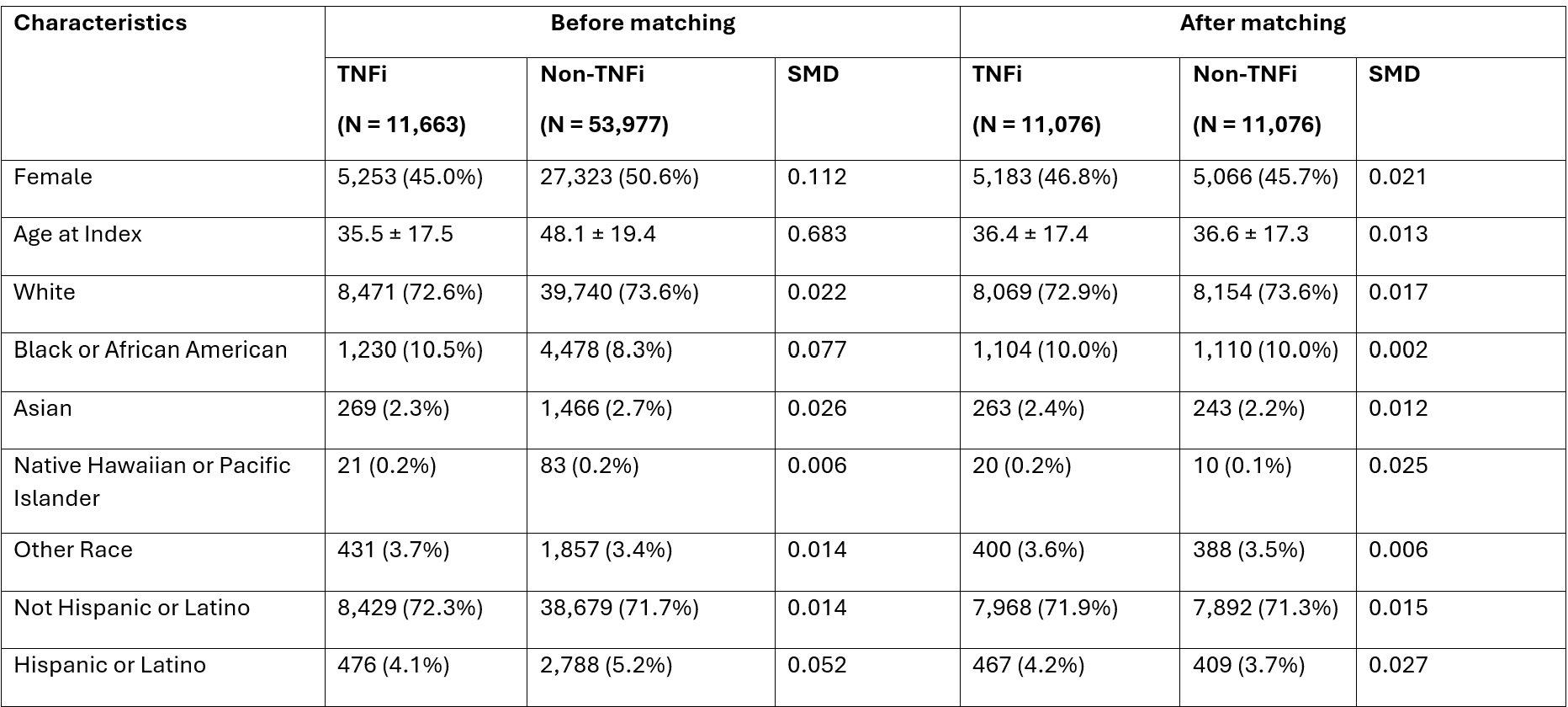
Figure: Figure 1. Adjusted Hazard Ratios for New-Onset Psoriasis and Psoriatic Arthritis Among Patients with IBD
Disclosures:
Omar Al Ta’ani indicated no relevant financial relationships.
Jana Hashash: BMS – Ad Board.
Yahya Alhalalmeh indicated no relevant financial relationships.
Zain Al Ta'ani indicated no relevant financial relationships.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Saqr Alsakarneh indicated no relevant financial relationships.
Omar Al Ta’ani, MD1, Jana G. Hashash, MD, MSc, FACG2, Yahya Alhalalmeh, MD3, Zain Al Ta'ani, MD4, Francis A.. Farraye, MD, MSc, MACG2, Saqr Alsakarneh, MD, MSc5. P5341 - A Multicenter Study Identifying the Risk of Psoriasis in Patients with Inflammatory Bowel Disease Treated with TNF-α Inhibitors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Internal Medicine, Allegheny Health Network, Pittsburgh, Pennsylvania, USA, Pittsburgh, PA; 2Mayo Clinic, Jacksonville, FL; 3New York Medical College - Saint Michael's Medical Center, Newark, NJ; 4University of Jordan, Amman, 'Amman, Jordan; 5Mayo Clinic, Rochester, MN
Introduction: Tumor necrosis factor-alpha (TNF-α) inhibitors are widely used in the treatment of inflammatory bowel disease (IBD), with proven efficacy in achieving and maintaining remission. However, these agents have been implicated in paradoxical immune-mediated adverse effects, including psoriasis. This study aimed to quantify the risk of incident psoriasis and psoriatic arthritis in patients with IBD receiving TNF-α inhibitor
Methods: We conducted a retrospective cohort study using the TriNetX research network, identifying adult patients with IBD who initiated treatment with either TNF-α inhibitors or non–TNF-α-based treatments. Propensity score matching (1:1) was applied to balance baseline characteristics, including age, sex, comorbidities, and IBD subtype. The primary outcome was a new diagnosis of psoriasis or psoriatic arthritis within five years of treatment initiation. Adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) were estimated using Cox proportional hazards models
Results: A total of 11,663 IBD patients treated with TNF-α inhibitors were compared to 53,977 patients receiving non–TNF-α therapies. After matching, 11,076 patients remained in each group with well-balanced characteristics (Table 1). TNF-α inhibitor use was associated with a significantly increased risk of psoriasis or psoriatic arthritis (aHR 1.63; 95% CI, 1.35–1.98; p < 0.001).
Subgroup analysis revealed significantly higher risk among females (aHR 1.90; 95% CI, 1.45–2.48; p < 0.001), while no significant association was seen in males (aHR 1.16; 95% CI, 0.85–1.58; p = 0.354). The greatest risk was observed in patients aged >60 years (aHR 2.56; 95% CI, 1.58–4.15; p < 0.001). Among individual agents, infliximab (aHR 1.53; 95% CI, 1.16–2.03; p = 0.003) and adalimumab (aHR 1.45; 95% CI, 1.09–1.92; p = 0.010) were both associated with elevated risk.
Upon stratification, both psoriasis (aHR 1.77; 95% CI, 1.46–2.14; p < 0.001) and psoriatic arthritis (aHR 1.54; 95% CI, 1.01–2.36; p = 0.048) showed significantly increased incidence in the TNF-α group, as shown in Figure 1
Discussion: In this study, TNF-α inhibitor therapy was associated with a significantly increased risk of new-onset psoriasis and psoriatic arthritis in patients with IBD. These findings underscore the importance of surveillance for paradoxical inflammatory manifestations and support individualized treatment decision-making in IBD management. Further studies are needed to better understand the mechanisms and inform strategies to minimize risk

Figure: Table 1. Baseline characteristics for patients who have IBD with TNFi medication or other IBD medication before and after propensity score
matching.

Figure: Figure 1. Adjusted Hazard Ratios for New-Onset Psoriasis and Psoriatic Arthritis Among Patients with IBD
Disclosures:
Omar Al Ta’ani indicated no relevant financial relationships.
Jana Hashash: BMS – Ad Board.
Yahya Alhalalmeh indicated no relevant financial relationships.
Zain Al Ta'ani indicated no relevant financial relationships.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Saqr Alsakarneh indicated no relevant financial relationships.
Omar Al Ta’ani, MD1, Jana G. Hashash, MD, MSc, FACG2, Yahya Alhalalmeh, MD3, Zain Al Ta'ani, MD4, Francis A.. Farraye, MD, MSc, MACG2, Saqr Alsakarneh, MD, MSc5. P5341 - A Multicenter Study Identifying the Risk of Psoriasis in Patients with Inflammatory Bowel Disease Treated with TNF-α Inhibitors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
