Tuesday Poster Session
Category: IBD
P5339 - A 5-Year Head-to-Head Safety Analysis of Adalimumab vs Infliximab in Inflammatory Bowel Disease
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- OA
Osama Alshakhatreh, MD (he/him/his)
Albany Medical Center
Norfolk, VA
Presenting Author(s)
Omar Arman, MD, MPH1, Lina Wahbeh, MD2, Osama Alshakhatreh, MD3, Abdulrahman Arman, MD4, Khaled Rafeh, MD5, Mazen Zamzam, BS6, Jad Bou-Abdallah, MD1
1University at Buffalo, Buffalo, NY; 2University of Jordan, Amman, 'Amman, Jordan; 3Albany Medical Center, Albany, NY; 4University of Jordan School of Medicine, Amman, 'Amman, Jordan; 5School of Medicine, The University of Jordan, Shmeisani, 'Amman, Jordan; 6Oakland University William Beaumont School of Medicine, Royal Oak, MI
Introduction: Adalimumab and infliximab are TNF inhibitors with proven efficacy in managing inflammatory bowel disease (IBD). Due to the chronic nature of IBD, TNF inhibitors are often indicated for long-term use. However, their immunosuppressive effects and influence on coagulation are associated with an increased risk of infections, cancer, thromboembolism (TE), and hospitalizations. While long-term safety data exist for these agents, direct comparisons of their safety profiles over extended periods remain limited. This study provides a comprehensive 5-year safety comparison of adalimumab and infliximab in IBD patients.
Methods: Our retrospective cohort study utilized the TriNetX database, a global federated health research network. Patients aged ≥18 years with a diagnosis of IBD who were treated with either adalimumab or infliximab were included. Propensity score matching was applied to balance baseline demographics and disease severity between the cohorts, yielding 28,912 matched patients in each group. Primary outcomes were the risks and risk difference between cohorts for IBD-related complications (infections, cancer, TE) and hospitalizations at 6mo, 1y, 3y, and 5y. Kaplan-Meier analysis was performed to evaluate event-free survival rates for each outcome.
Results: Over 5 years, cancer incidence remained low and similar between the adalimumab and infliximab groups, with rates under 0.2% (p = 0.925). Adalimumab consistently demonstrated lower infection rates compared to infliximab (5.61% vs. 7.72% at 6 months, 8.1% vs. 10.3% at 1 year, and 16.1% vs. 18.0% at 5 years; p < 0.001). The risk of TE was also lower for adalimumab (1.42% vs. 2.15% at 6 months, decreasing to 3.4% vs. 3.7% at 5 years; p = 0.014). Hospitalization rates showed a similar trend favoring adalimumab, from 6.61% vs. 11.62% at 6 months to 14.7% vs. 19.1% at 5 years (p < 0.001).
Discussion: Over five years, adalimumab demonstrated significantly lower rates of infections, TE, and hospitalizations compared to infliximab, while cancer incidence remained comparable between the two therapies. These findings suggest that adalimumab may offer a safer long-term profile, reducing the risks of severe complications and alleviating healthcare burdens through fewer hospitalizations. Clinicians should consider these safety differences when personalizing treatment strategies for IBD patients. Future prospective studies are warranted to confirm these findings and explore the mechanisms driving these differences.
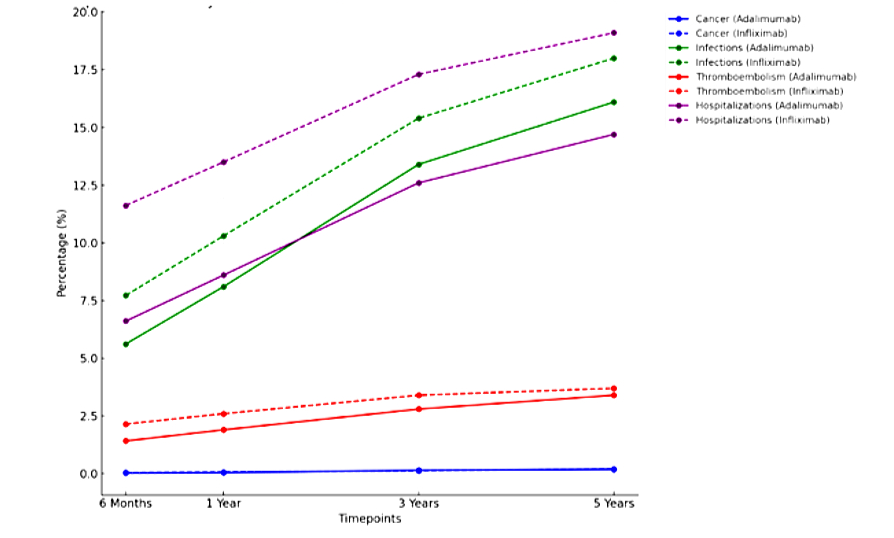
Figure: Table 1: Long-Term Safety Comparison of Adalimumab vs. Infliximab in IBD Patients
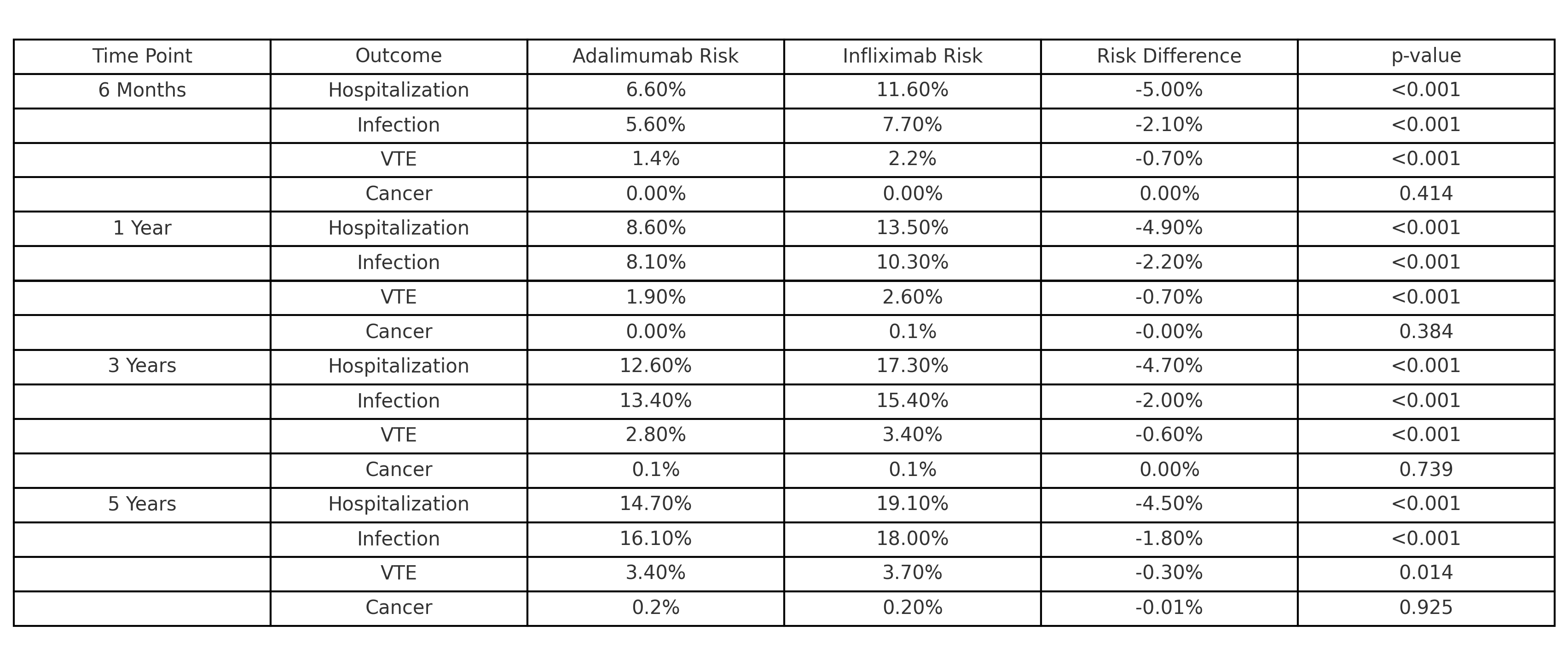
Figure: Figure 1: Comparison of Safety Outcomes Between Adalimumab and Infliximab Over 5 Years in terms of cancer, infection, thromboembolism, and hospitalization rates.
Disclosures:
Omar Arman indicated no relevant financial relationships.
Lina Wahbeh indicated no relevant financial relationships.
Osama Alshakhatreh indicated no relevant financial relationships.
Abdulrahman Arman indicated no relevant financial relationships.
Khaled Rafeh indicated no relevant financial relationships.
Mazen Zamzam indicated no relevant financial relationships.
Jad Bou-Abdallah indicated no relevant financial relationships.
Omar Arman, MD, MPH1, Lina Wahbeh, MD2, Osama Alshakhatreh, MD3, Abdulrahman Arman, MD4, Khaled Rafeh, MD5, Mazen Zamzam, BS6, Jad Bou-Abdallah, MD1. P5339 - A 5-Year Head-to-Head Safety Analysis of Adalimumab vs Infliximab in Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University at Buffalo, Buffalo, NY; 2University of Jordan, Amman, 'Amman, Jordan; 3Albany Medical Center, Albany, NY; 4University of Jordan School of Medicine, Amman, 'Amman, Jordan; 5School of Medicine, The University of Jordan, Shmeisani, 'Amman, Jordan; 6Oakland University William Beaumont School of Medicine, Royal Oak, MI
Introduction: Adalimumab and infliximab are TNF inhibitors with proven efficacy in managing inflammatory bowel disease (IBD). Due to the chronic nature of IBD, TNF inhibitors are often indicated for long-term use. However, their immunosuppressive effects and influence on coagulation are associated with an increased risk of infections, cancer, thromboembolism (TE), and hospitalizations. While long-term safety data exist for these agents, direct comparisons of their safety profiles over extended periods remain limited. This study provides a comprehensive 5-year safety comparison of adalimumab and infliximab in IBD patients.
Methods: Our retrospective cohort study utilized the TriNetX database, a global federated health research network. Patients aged ≥18 years with a diagnosis of IBD who were treated with either adalimumab or infliximab were included. Propensity score matching was applied to balance baseline demographics and disease severity between the cohorts, yielding 28,912 matched patients in each group. Primary outcomes were the risks and risk difference between cohorts for IBD-related complications (infections, cancer, TE) and hospitalizations at 6mo, 1y, 3y, and 5y. Kaplan-Meier analysis was performed to evaluate event-free survival rates for each outcome.
Results: Over 5 years, cancer incidence remained low and similar between the adalimumab and infliximab groups, with rates under 0.2% (p = 0.925). Adalimumab consistently demonstrated lower infection rates compared to infliximab (5.61% vs. 7.72% at 6 months, 8.1% vs. 10.3% at 1 year, and 16.1% vs. 18.0% at 5 years; p < 0.001). The risk of TE was also lower for adalimumab (1.42% vs. 2.15% at 6 months, decreasing to 3.4% vs. 3.7% at 5 years; p = 0.014). Hospitalization rates showed a similar trend favoring adalimumab, from 6.61% vs. 11.62% at 6 months to 14.7% vs. 19.1% at 5 years (p < 0.001).
Discussion: Over five years, adalimumab demonstrated significantly lower rates of infections, TE, and hospitalizations compared to infliximab, while cancer incidence remained comparable between the two therapies. These findings suggest that adalimumab may offer a safer long-term profile, reducing the risks of severe complications and alleviating healthcare burdens through fewer hospitalizations. Clinicians should consider these safety differences when personalizing treatment strategies for IBD patients. Future prospective studies are warranted to confirm these findings and explore the mechanisms driving these differences.

Figure: Table 1: Long-Term Safety Comparison of Adalimumab vs. Infliximab in IBD Patients

Figure: Figure 1: Comparison of Safety Outcomes Between Adalimumab and Infliximab Over 5 Years in terms of cancer, infection, thromboembolism, and hospitalization rates.
Disclosures:
Omar Arman indicated no relevant financial relationships.
Lina Wahbeh indicated no relevant financial relationships.
Osama Alshakhatreh indicated no relevant financial relationships.
Abdulrahman Arman indicated no relevant financial relationships.
Khaled Rafeh indicated no relevant financial relationships.
Mazen Zamzam indicated no relevant financial relationships.
Jad Bou-Abdallah indicated no relevant financial relationships.
Omar Arman, MD, MPH1, Lina Wahbeh, MD2, Osama Alshakhatreh, MD3, Abdulrahman Arman, MD4, Khaled Rafeh, MD5, Mazen Zamzam, BS6, Jad Bou-Abdallah, MD1. P5339 - A 5-Year Head-to-Head Safety Analysis of Adalimumab vs Infliximab in Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
