Tuesday Poster Session
Category: IBD
P5335 - JAK Inhibitors for Crohn's Disease: A Systematic Review and Network Meta-Analysis of Efficacy and Safety
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Fares Qtaishat (he/him/his)
University of Jordan School of Medicine
Amman, 'Amman, Jordan
Presenting Author(s)
Fares Qtaishat, 1, Mohammad-Amer Tamimi, 1, Ahmad AlKayyat, 1, Jehad Yasin, 1, Jana Tarawneh, 1, Layan AlDaher, 1, Sarah Abdallah, 2, Mohammad Alghaniem, 1, Yousef Taha, 3, Abdallah Abunamoos, 1
1University of Jordan School of Medicine, Amman, 'Amman, Jordan; 2Istanbul Medipol University, Istanbul, Istanbul, Turkey; 3The American University in Cairo, Cairo, Al Qahirah, Egypt
Introduction: Crohn’s disease (CD) is a chronic inflammatory condition with limited effective treatment options. Janus Kinase (JAK) inhibitors offer a novel approach but lack direct comparative data in CD. This study conducts a dose-dependent network meta-analysis of randomized controlled trials (RCTs) to compare various JAK inhibitors.
Methods: PubMed, Cochrane Library, Scopus, and Web of Science were systematically searched from inception to February 17, 2025. RCTs assessing the efficacy and safety of JAK inhibitors in the treatment of adults with Crohn’s disease were included. Dose-response Bayesian network meta-analysis utilizing an Emax model was conducted using “MBNMA” package in R, and treatment effects were estimated using risk ratios (RRs) and 95% credible intervals (CrIs). Risk of bias was assessed with RoB 2.
Results: Nine RCTs (n = 4838) evaluated Upadacitinib (UPA), Tofacitinib (TOFA), and Filgotinib (FIL) at multiple doses. Upadacitinib 45 mg once daily demonstrated the greatest benefit in achieving CDAI (Crohn’s Disease Activity Index) remission compared to placebo (RR = 1.8, 95% CrI [1.1–3.0]). SUCRA rankings showed that UPA ranked highest for overall clinical and CDAI remission. Meta-regression revealed a statistically significant association between higher doses and increased remission rates (p = 0.041). According to dose–response modeling, UPA had both a higher Emax and a lower ED50, indicating greater efficacy and potency compared to TOFA and FIL. Serious adverse events (AEs) were significantly more common with JAK inhibitors compared to placebo (RR = 1.61; 95% CI: 1.15–2.25; p = 0.0082). Specific events such as sepsis, cardiovascular AEs, and elevated liver enzymes occurred more frequently in the JAK inhibitor group (p < 0.05). Subgroup analysis revealed significant differences in the risk of overall AEs among individual JAK inhibitors assessed (p = 0.0008), with UPA showing higher AE rates compared to placebo (RR = 1.19; 95% CI: 1.07–1.33). Additionally, UPA significantly increased the risk of serious infections compared to placebo (RR = 5.39; 95% CI: 2.98–9.74).
Discussion: JAK inhibitors significantly improved clinical outcomes in Crohn’s disease patients. UPA demonstrated the most consistent efficacy across trials, characterized by a favorable pharmacodynamic profile compared to TOFA and FIL. However, these therapeutic gains of UPA were accompanied by a higher incidence of adverse events, particularly serious infections.
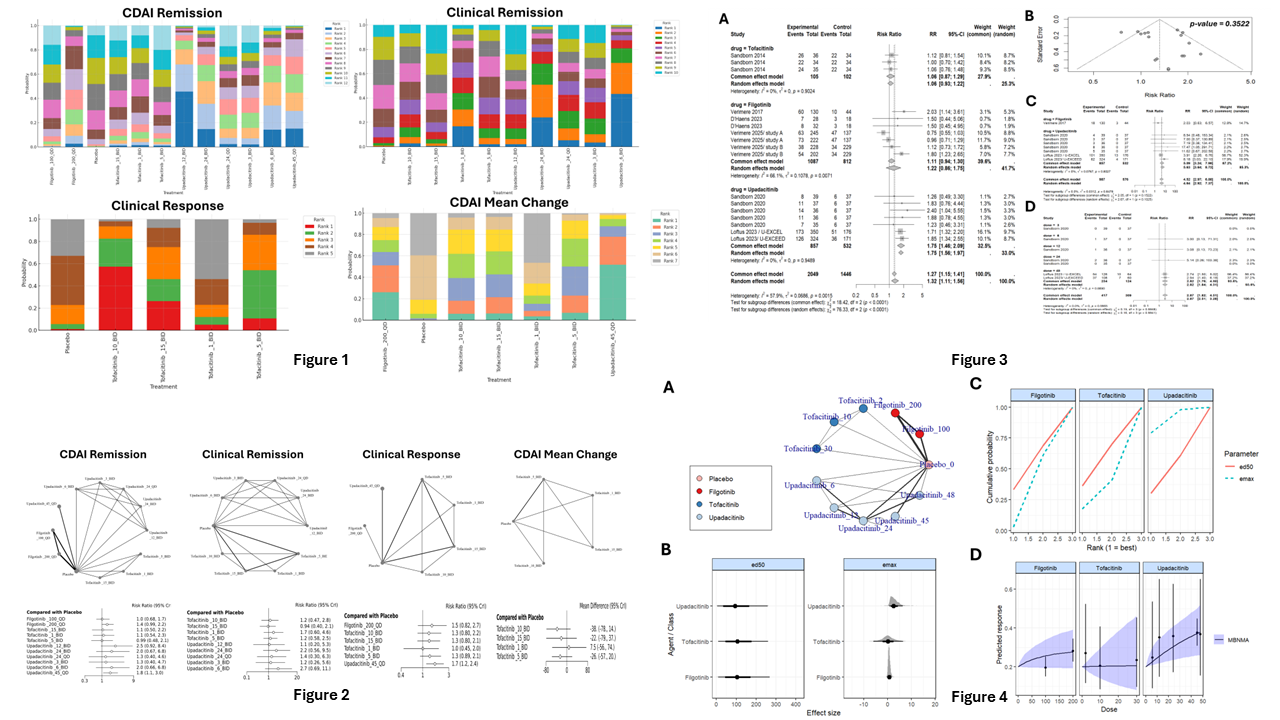
Figure: Surface Under the Cumulative Ranking curve (SUCRA) analysis of tofacitinib, filgotinib, and upadacitinib illustrating CDAI remission, clinical remission, clinical response, and CDAI mean change outcomes through rank probability bar charts (Figure 1), corresponding network diagrams and forest plots (Figure 2), pooled effect sizes with risk ratios and heterogeneity assessments (Figure 3), and dose–response and pharmacodynamic curves for each JAK inhibitor (Figure 4)
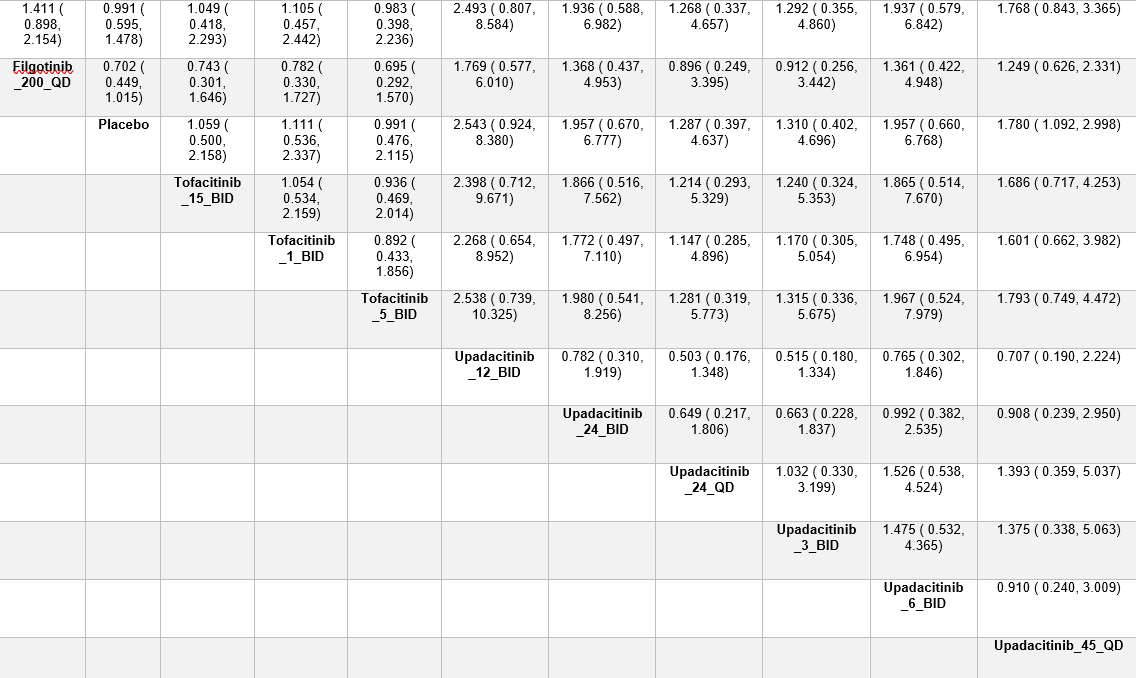
Figure: League table showing risk ratios for achieving CDAI remission with different doses of Filgotinib, Tofacitinib, Upadacitinib, and placebo.
Disclosures:
Fares Qtaishat indicated no relevant financial relationships.
Mohammad-Amer Tamimi indicated no relevant financial relationships.
Ahmad AlKayyat indicated no relevant financial relationships.
Jehad Yasin indicated no relevant financial relationships.
Jana Tarawneh indicated no relevant financial relationships.
Layan AlDaher indicated no relevant financial relationships.
Sarah Abdallah indicated no relevant financial relationships.
Mohammad Alghaniem indicated no relevant financial relationships.
Yousef Taha indicated no relevant financial relationships.
Abdallah Abunamoos indicated no relevant financial relationships.
Fares Qtaishat, 1, Mohammad-Amer Tamimi, 1, Ahmad AlKayyat, 1, Jehad Yasin, 1, Jana Tarawneh, 1, Layan AlDaher, 1, Sarah Abdallah, 2, Mohammad Alghaniem, 1, Yousef Taha, 3, Abdallah Abunamoos, 1. P5335 - JAK Inhibitors for Crohn's Disease: A Systematic Review and Network Meta-Analysis of Efficacy and Safety, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Jordan School of Medicine, Amman, 'Amman, Jordan; 2Istanbul Medipol University, Istanbul, Istanbul, Turkey; 3The American University in Cairo, Cairo, Al Qahirah, Egypt
Introduction: Crohn’s disease (CD) is a chronic inflammatory condition with limited effective treatment options. Janus Kinase (JAK) inhibitors offer a novel approach but lack direct comparative data in CD. This study conducts a dose-dependent network meta-analysis of randomized controlled trials (RCTs) to compare various JAK inhibitors.
Methods: PubMed, Cochrane Library, Scopus, and Web of Science were systematically searched from inception to February 17, 2025. RCTs assessing the efficacy and safety of JAK inhibitors in the treatment of adults with Crohn’s disease were included. Dose-response Bayesian network meta-analysis utilizing an Emax model was conducted using “MBNMA” package in R, and treatment effects were estimated using risk ratios (RRs) and 95% credible intervals (CrIs). Risk of bias was assessed with RoB 2.
Results: Nine RCTs (n = 4838) evaluated Upadacitinib (UPA), Tofacitinib (TOFA), and Filgotinib (FIL) at multiple doses. Upadacitinib 45 mg once daily demonstrated the greatest benefit in achieving CDAI (Crohn’s Disease Activity Index) remission compared to placebo (RR = 1.8, 95% CrI [1.1–3.0]). SUCRA rankings showed that UPA ranked highest for overall clinical and CDAI remission. Meta-regression revealed a statistically significant association between higher doses and increased remission rates (p = 0.041). According to dose–response modeling, UPA had both a higher Emax and a lower ED50, indicating greater efficacy and potency compared to TOFA and FIL. Serious adverse events (AEs) were significantly more common with JAK inhibitors compared to placebo (RR = 1.61; 95% CI: 1.15–2.25; p = 0.0082). Specific events such as sepsis, cardiovascular AEs, and elevated liver enzymes occurred more frequently in the JAK inhibitor group (p < 0.05). Subgroup analysis revealed significant differences in the risk of overall AEs among individual JAK inhibitors assessed (p = 0.0008), with UPA showing higher AE rates compared to placebo (RR = 1.19; 95% CI: 1.07–1.33). Additionally, UPA significantly increased the risk of serious infections compared to placebo (RR = 5.39; 95% CI: 2.98–9.74).
Discussion: JAK inhibitors significantly improved clinical outcomes in Crohn’s disease patients. UPA demonstrated the most consistent efficacy across trials, characterized by a favorable pharmacodynamic profile compared to TOFA and FIL. However, these therapeutic gains of UPA were accompanied by a higher incidence of adverse events, particularly serious infections.

Figure: Surface Under the Cumulative Ranking curve (SUCRA) analysis of tofacitinib, filgotinib, and upadacitinib illustrating CDAI remission, clinical remission, clinical response, and CDAI mean change outcomes through rank probability bar charts (Figure 1), corresponding network diagrams and forest plots (Figure 2), pooled effect sizes with risk ratios and heterogeneity assessments (Figure 3), and dose–response and pharmacodynamic curves for each JAK inhibitor (Figure 4)

Figure: League table showing risk ratios for achieving CDAI remission with different doses of Filgotinib, Tofacitinib, Upadacitinib, and placebo.
Disclosures:
Fares Qtaishat indicated no relevant financial relationships.
Mohammad-Amer Tamimi indicated no relevant financial relationships.
Ahmad AlKayyat indicated no relevant financial relationships.
Jehad Yasin indicated no relevant financial relationships.
Jana Tarawneh indicated no relevant financial relationships.
Layan AlDaher indicated no relevant financial relationships.
Sarah Abdallah indicated no relevant financial relationships.
Mohammad Alghaniem indicated no relevant financial relationships.
Yousef Taha indicated no relevant financial relationships.
Abdallah Abunamoos indicated no relevant financial relationships.
Fares Qtaishat, 1, Mohammad-Amer Tamimi, 1, Ahmad AlKayyat, 1, Jehad Yasin, 1, Jana Tarawneh, 1, Layan AlDaher, 1, Sarah Abdallah, 2, Mohammad Alghaniem, 1, Yousef Taha, 3, Abdallah Abunamoos, 1. P5335 - JAK Inhibitors for Crohn's Disease: A Systematic Review and Network Meta-Analysis of Efficacy and Safety, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
