Tuesday Poster Session
Category: IBD
P5334 - Efficacy and Safety of Mirikizumab in the Treatment of Moderate to Severe Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Joel Gabin Konlack Mekontso, MD (he/him/his)
New York City Health and Hospitals, South Brooklyn Health
New York, NY
Presenting Author(s)
Joel Gabin Konlack Mekontso, MD1, Joseph Yvan Bena Nnang, 2, Samuel Ghislain Junior Fodop, 3, Bishoy Ibrahim Boulus Elkoumes, MD4, Guy Loic Nguefang Tchoukeu, MD5, Anifatou Berinyuy Kortim, MD6, Ticha Brandon Tita Tembi, MD, MPH7, Akil Olliverre, MD4, Jarin Prasa, DO4, John Trillo, MD8
1New York City Health and Hospitals, South Brooklyn Health, New York, NY; 2University of Yaounde 1, Faculty of Medicine and Biomedical Sciences, Yaounde, Centre, Cameroon; 3Catholic University, School of Health and Medical Sciences, Kumbo, Nord-Ouest, Cameroon; 4NYC Health + Hospitals/South Brooklyn Health, New York, NY; 5Texas Tech University Health Sciences Center, Odessa, TX; 6University of Bamenda, Faculty of Health Sciences, Bamenda, Nord-Ouest, Cameroon; 7Emory University, Atlanta, GA; 8NYC Health + Hospitals/South Brooklyn Health, Brooklyn, NY
Introduction: Inflammatory bowel diseases (IBD), including Ulcerative colitis (UC) and Crohn’s disease (CD) remain challenging to treat despite advances. Mirikizumab, a monoclonal antibody that selectively inhibits the p19 subunit of Interleukin-23, shows promise. This study consolidated available evidence to assess its efficacy and safety across both IBD populations.
Methods: We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) comparing Mirikizumab to placebo in moderate-to-severe IBD. We assessed clinical response, clinical remission, endoscopic remission, adverse events (AEs), and treatment discontinuation. We applied a random-effects model to calculate risk ratios (RR) with 95% confidence intervals (CI) and reported numbers needed to treat (NNT) and harm (NNH).
Results: We included 4 RCTs (2,380 patients). Mirikizumab increased the likelihood of clinical response by 69% (61.0% vs. 36.6%; RR 1.69; 95% CI [1.35, 2.10]; p < 0.00001; NNT = 4), clinical remission by 74% (37.1% vs. 20.0%; RR 1.74; 95% CI [1.50, 2.02]; p < 0.00001; NNT = 7), and endoscopic remission by 92% (28.7% vs. 15.4%; RR 1.92; 95% CI [1.63, 2.27]; p < 0.00001; NNT = 8).
Mirikizumab-induced clinical and endoscopic remission extended to both the induction and maintenance phases of treatment and across UC and CD populations, with biologic-failed patients also experiencing significantly higher clinical response rates compared to placebo (62.0% vs. 35.8%; RR 1.75; 95% CI [1.56, 1.96]; p < 0.00001).
Mirikizumab was safe, with reduced rates of serious AEs (5.2% vs. 9.1%; RR 0.53; 95% CI [0.41, 0.70]; p< 0.00001; NNH = 24), treatment discontinuation (2.5% vs. 7.5%; RR 0.33; 95% CI [0.20, 0.56]; p < 0.0001; NNH = 20), and IBD worsening (3.3% vs. 13%; RR 0.26; 95% CI [0.19, 0.36]; p < 0.00001; NNT = 11).
Discussion: Mirikizumab is effective in achieving clinical and endoscopic remission in IBD, including in biologic-experienced patients. Its favorable safety profile, with reduced serious AEs and discontinuations, aligns with its selective mechanism. While higher induction doses appear necessary, maintenance efficacy is robust, with flexible administration routes. Our findings support Mirikizumab as a valuable first or second-line treatment for moderate-to-severe IBD. Future research should focus on long-term outcomes, real-world data, diverse populations, and dose optimization, while prioritizing consistent outcome reporting, to further highlight Mirikizumab's clinical utility.
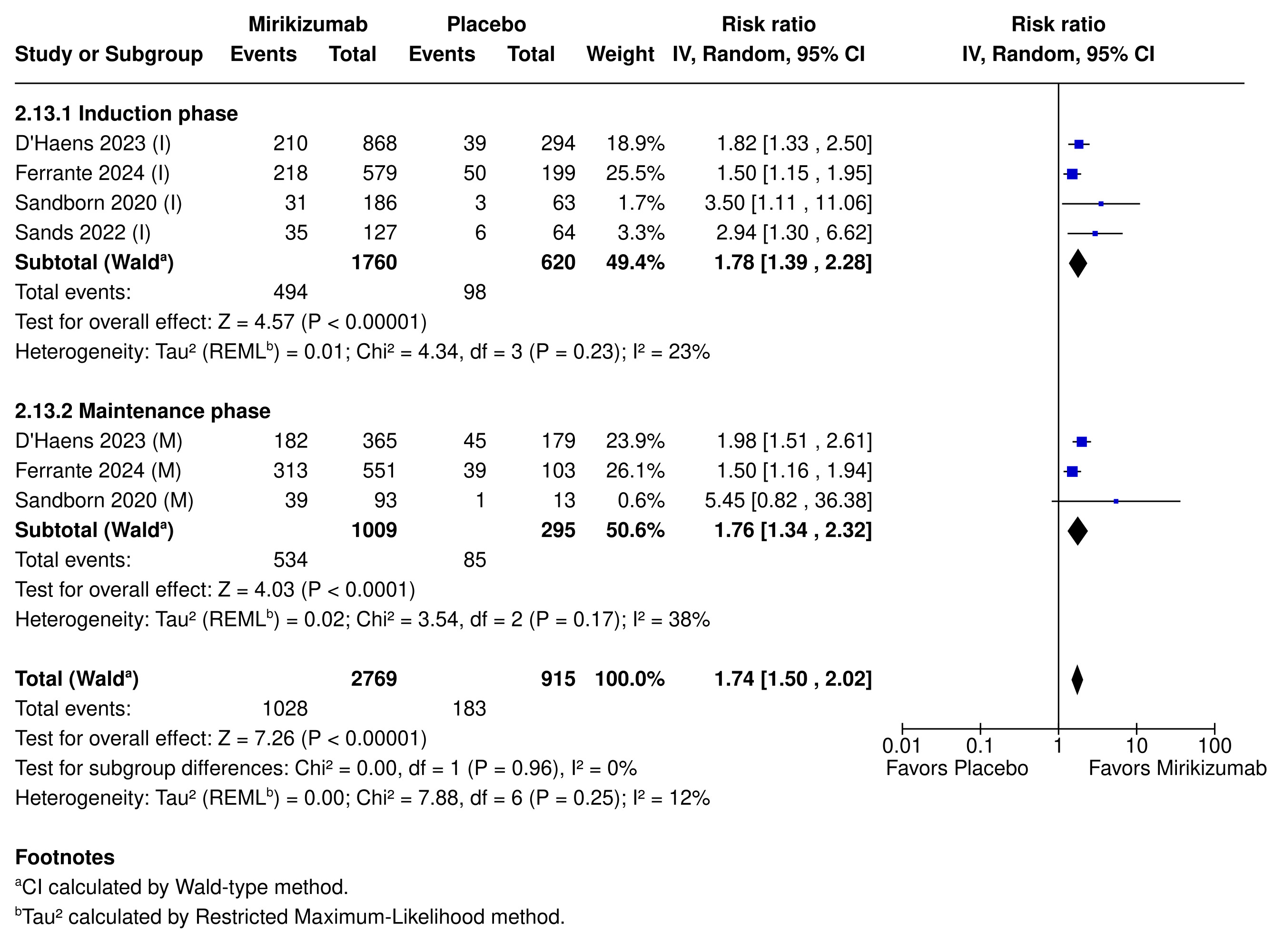
Figure: Figure 1: Clinical remission rates in patients treated with Mirikizumab during induction (I) and maintenance (M) phases.
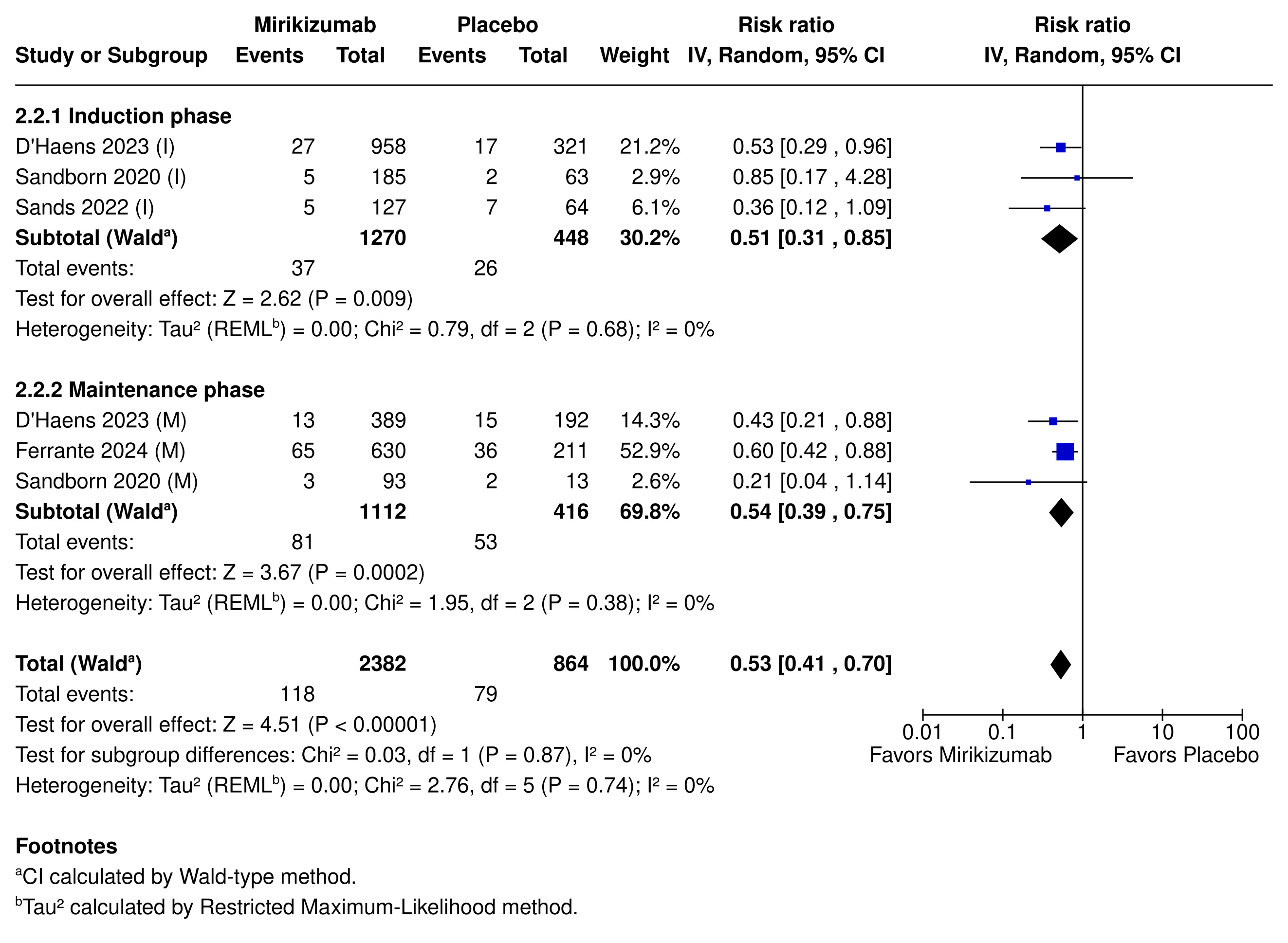
Figure: Figure 2: Incidence of serious adverse events in patients treated with Mirikizumab compared to placebo during induction (I) and maintenance (M) phases.
Disclosures:
Joel Gabin Konlack Mekontso indicated no relevant financial relationships.
Joseph Yvan Bena Nnang indicated no relevant financial relationships.
Samuel Ghislain Junior Fodop indicated no relevant financial relationships.
Bishoy Ibrahim Boulus Elkoumes indicated no relevant financial relationships.
Guy Loic Nguefang Tchoukeu indicated no relevant financial relationships.
Anifatou Berinyuy Kortim indicated no relevant financial relationships.
Ticha Brandon Tita Tembi indicated no relevant financial relationships.
Akil Olliverre indicated no relevant financial relationships.
Jarin Prasa indicated no relevant financial relationships.
John Trillo indicated no relevant financial relationships.
Joel Gabin Konlack Mekontso, MD1, Joseph Yvan Bena Nnang, 2, Samuel Ghislain Junior Fodop, 3, Bishoy Ibrahim Boulus Elkoumes, MD4, Guy Loic Nguefang Tchoukeu, MD5, Anifatou Berinyuy Kortim, MD6, Ticha Brandon Tita Tembi, MD, MPH7, Akil Olliverre, MD4, Jarin Prasa, DO4, John Trillo, MD8. P5334 - Efficacy and Safety of Mirikizumab in the Treatment of Moderate to Severe Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1New York City Health and Hospitals, South Brooklyn Health, New York, NY; 2University of Yaounde 1, Faculty of Medicine and Biomedical Sciences, Yaounde, Centre, Cameroon; 3Catholic University, School of Health and Medical Sciences, Kumbo, Nord-Ouest, Cameroon; 4NYC Health + Hospitals/South Brooklyn Health, New York, NY; 5Texas Tech University Health Sciences Center, Odessa, TX; 6University of Bamenda, Faculty of Health Sciences, Bamenda, Nord-Ouest, Cameroon; 7Emory University, Atlanta, GA; 8NYC Health + Hospitals/South Brooklyn Health, Brooklyn, NY
Introduction: Inflammatory bowel diseases (IBD), including Ulcerative colitis (UC) and Crohn’s disease (CD) remain challenging to treat despite advances. Mirikizumab, a monoclonal antibody that selectively inhibits the p19 subunit of Interleukin-23, shows promise. This study consolidated available evidence to assess its efficacy and safety across both IBD populations.
Methods: We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) comparing Mirikizumab to placebo in moderate-to-severe IBD. We assessed clinical response, clinical remission, endoscopic remission, adverse events (AEs), and treatment discontinuation. We applied a random-effects model to calculate risk ratios (RR) with 95% confidence intervals (CI) and reported numbers needed to treat (NNT) and harm (NNH).
Results: We included 4 RCTs (2,380 patients). Mirikizumab increased the likelihood of clinical response by 69% (61.0% vs. 36.6%; RR 1.69; 95% CI [1.35, 2.10]; p < 0.00001; NNT = 4), clinical remission by 74% (37.1% vs. 20.0%; RR 1.74; 95% CI [1.50, 2.02]; p < 0.00001; NNT = 7), and endoscopic remission by 92% (28.7% vs. 15.4%; RR 1.92; 95% CI [1.63, 2.27]; p < 0.00001; NNT = 8).
Mirikizumab-induced clinical and endoscopic remission extended to both the induction and maintenance phases of treatment and across UC and CD populations, with biologic-failed patients also experiencing significantly higher clinical response rates compared to placebo (62.0% vs. 35.8%; RR 1.75; 95% CI [1.56, 1.96]; p < 0.00001).
Mirikizumab was safe, with reduced rates of serious AEs (5.2% vs. 9.1%; RR 0.53; 95% CI [0.41, 0.70]; p< 0.00001; NNH = 24), treatment discontinuation (2.5% vs. 7.5%; RR 0.33; 95% CI [0.20, 0.56]; p < 0.0001; NNH = 20), and IBD worsening (3.3% vs. 13%; RR 0.26; 95% CI [0.19, 0.36]; p < 0.00001; NNT = 11).
Discussion: Mirikizumab is effective in achieving clinical and endoscopic remission in IBD, including in biologic-experienced patients. Its favorable safety profile, with reduced serious AEs and discontinuations, aligns with its selective mechanism. While higher induction doses appear necessary, maintenance efficacy is robust, with flexible administration routes. Our findings support Mirikizumab as a valuable first or second-line treatment for moderate-to-severe IBD. Future research should focus on long-term outcomes, real-world data, diverse populations, and dose optimization, while prioritizing consistent outcome reporting, to further highlight Mirikizumab's clinical utility.

Figure: Figure 1: Clinical remission rates in patients treated with Mirikizumab during induction (I) and maintenance (M) phases.

Figure: Figure 2: Incidence of serious adverse events in patients treated with Mirikizumab compared to placebo during induction (I) and maintenance (M) phases.
Disclosures:
Joel Gabin Konlack Mekontso indicated no relevant financial relationships.
Joseph Yvan Bena Nnang indicated no relevant financial relationships.
Samuel Ghislain Junior Fodop indicated no relevant financial relationships.
Bishoy Ibrahim Boulus Elkoumes indicated no relevant financial relationships.
Guy Loic Nguefang Tchoukeu indicated no relevant financial relationships.
Anifatou Berinyuy Kortim indicated no relevant financial relationships.
Ticha Brandon Tita Tembi indicated no relevant financial relationships.
Akil Olliverre indicated no relevant financial relationships.
Jarin Prasa indicated no relevant financial relationships.
John Trillo indicated no relevant financial relationships.
Joel Gabin Konlack Mekontso, MD1, Joseph Yvan Bena Nnang, 2, Samuel Ghislain Junior Fodop, 3, Bishoy Ibrahim Boulus Elkoumes, MD4, Guy Loic Nguefang Tchoukeu, MD5, Anifatou Berinyuy Kortim, MD6, Ticha Brandon Tita Tembi, MD, MPH7, Akil Olliverre, MD4, Jarin Prasa, DO4, John Trillo, MD8. P5334 - Efficacy and Safety of Mirikizumab in the Treatment of Moderate to Severe Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
