Tuesday Poster Session
Category: IBD
P5332 - Impact of GLP-1 Receptor Agonist or GIP/GLP-1 Receptor Agonist on Clinical Outcomes in Obese Patients With Inflammatory Bowel Disease: A Propensity-Matched Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Mohamed Nadeem, MD
Cleveland Clinic Foundation
Fairview Park, OH
Presenting Author(s)
Mohamed Nadeem, MD1, Akash Khurana, MD2, Stephen Firkins, MD2, Roberto Simons-Linares, MD2
1Cleveland Clinic Foundation, Fairview Park, OH; 2Gastroenterology and Hepatology, Cleveland Clinic, Cleveland, OH
Introduction: Obesity affects around 20-40% inflammatory bowel disease (IBD) patients in Western countries. Glucagon-like peptide-1 receptor agonists (GLP-1RA) alone or a combination with glucose-dependent insulinotropic polypeptide receptor co-agonists (GLP-1/GIP RA) are effective for obesity, but their impact on IBD outcomes remain unclear. We aimed to assess the clinical impact of these medications in obese IBD patients.
Methods: We conducted a retrospective, propensity score-matched cohort study using TriNetX, a global federated research network. Adults with IBD and obesity (BMI ≥30) were matched 1:1 based on demographics, commodities, and baseline medication use, comparing those on GLP-1RA (Semaglutide/dulaglutide/liraglutide) or GIP/GLP-1RA (Tirzepatide) to those not on these medications. The primary composite outcome included all-cause hospitalization, steroid use, colectomy, initiation of biologic (Vedolizumab, Ustekinumab, Risankizumab), and initiation of small molecules (Upadacitinib, tofacitinib) over 10 years. Secondary outcomes included each component individually, all-cause mortality, elevated CRP≥ 10 mg/L, fecal calprotectin ≥ 50 ug/g, acute pancreatitis, and ED visits. Subgroup analysis with just Crohn’s disease (CD) and ulcerative colitis (UC) were done. Odds ratios (ORs) with 95% confidence intervals (CIs) and p-values were reported.
Results: In the matched IBD cohort (n=5,772 per group), GLP-1RA or GIP/GLP-1RA use was associated with significantly reduced risks of primary composite outcome (51% vs. 65%, OR 0.562, CI 0.522-0.606; p< 0.001), risk of hospitalization (27% vs. 43.2%), all-cause mortality (2.8% vs.12.1%), steroid use (41.2% vs 49.9%), colectomy (0.4% vs. 0.9%), ED visits (35.9% vs. 45.4%). CRP (13.7% vs. 24.1%) and fecal calprotectin (5.5% vs. 7%) elevations were higher in the non-GLP-1 RA or GIP/GLP-1 RA group. However, biologic use was more in the GLP-1RA or GIP/GLP-1 RA group (2.5% vs.1.7%; OR 1.477; p=0.003). Rates of acute pancreatitis were similar(p=0.92). Subgroup analysis in CD and UC showed consistent findings, with reduced composite outcome risk in both. No significant difference in biologic use was seen in subgroups.
Discussion: GLP-1 RA or GIP/GLP-1 RA use in IBD patients with obesity is associated with significantly improved clinical outcomes such as hospitalization, mortality, steroid use and inflammatory burden. These findings highlight a potential therapeutic benefit in modifying disease course in obese IBD patients and warrant further prospective studies.
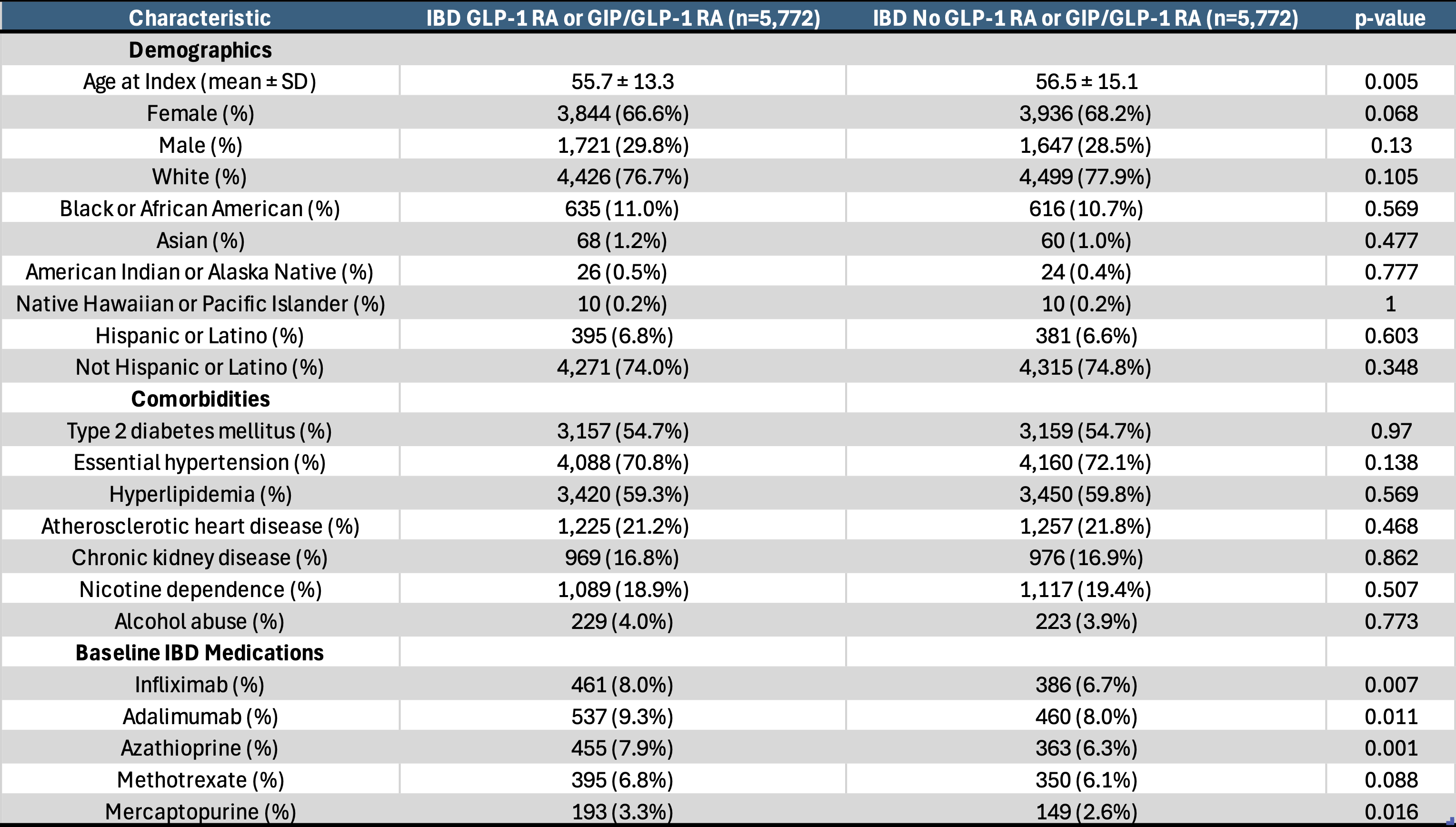
Figure: Baseline characteristics after matching in IBD patients with obesity cohort with or without GLP-1 RA or GIP/GLP-1 RA use
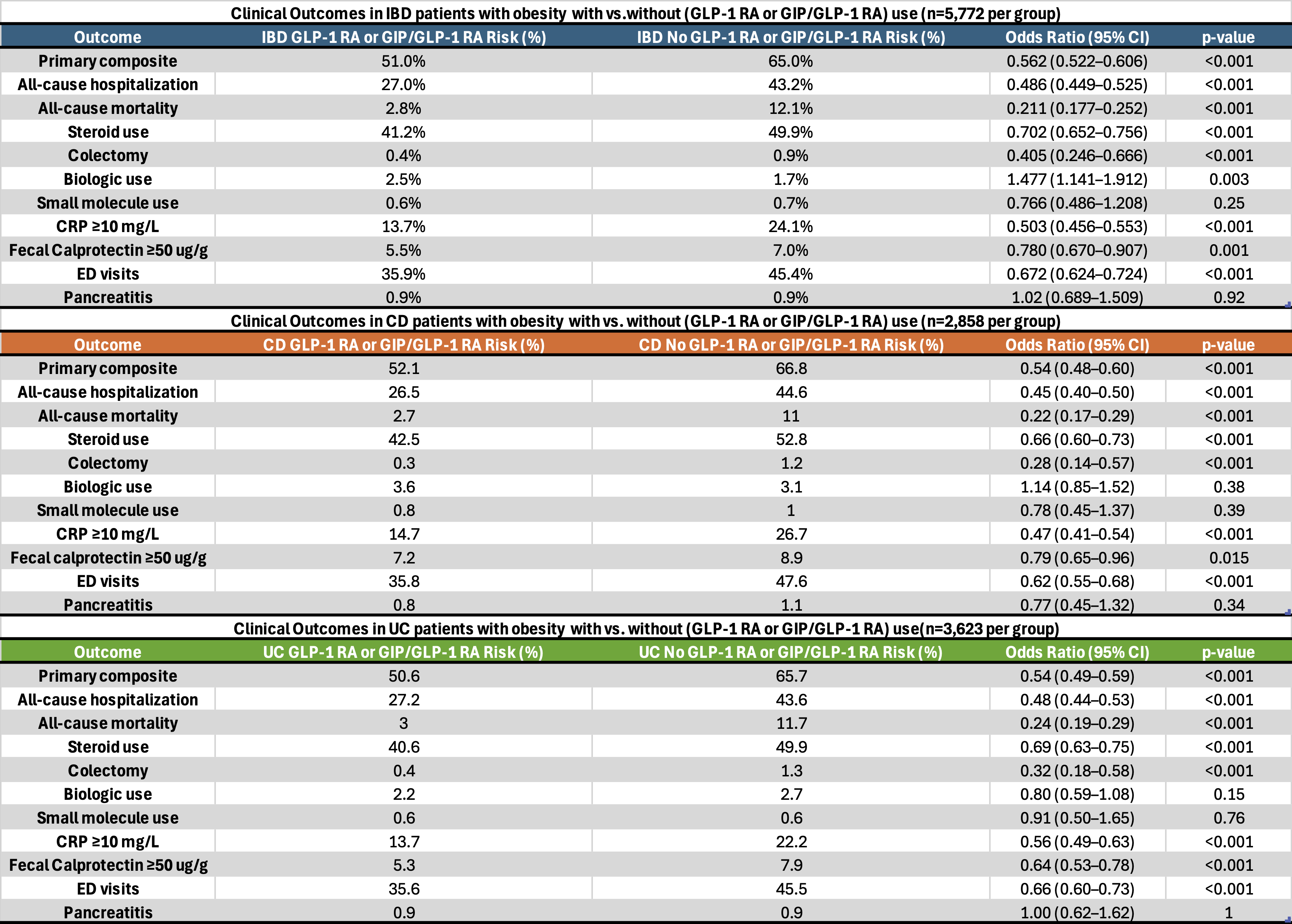
Figure: Table 2: Clinical outcomes in IBD patients with obesity with vs. without GLP-1 RA or GIP/GLP-1 RA use. Subgroup analysis with UC patients with obesity and CD patients with obesity with vs. without GLP-1 RA or GIP/GLP-1 RA use
Disclosures:
Mohamed Nadeem indicated no relevant financial relationships.
Akash Khurana indicated no relevant financial relationships.
Stephen Firkins indicated no relevant financial relationships.
Roberto Simons-Linares indicated no relevant financial relationships.
Mohamed Nadeem, MD1, Akash Khurana, MD2, Stephen Firkins, MD2, Roberto Simons-Linares, MD2. P5332 - Impact of GLP-1 Receptor Agonist or GIP/GLP-1 Receptor Agonist on Clinical Outcomes in Obese Patients With Inflammatory Bowel Disease: A Propensity-Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Cleveland Clinic Foundation, Fairview Park, OH; 2Gastroenterology and Hepatology, Cleveland Clinic, Cleveland, OH
Introduction: Obesity affects around 20-40% inflammatory bowel disease (IBD) patients in Western countries. Glucagon-like peptide-1 receptor agonists (GLP-1RA) alone or a combination with glucose-dependent insulinotropic polypeptide receptor co-agonists (GLP-1/GIP RA) are effective for obesity, but their impact on IBD outcomes remain unclear. We aimed to assess the clinical impact of these medications in obese IBD patients.
Methods: We conducted a retrospective, propensity score-matched cohort study using TriNetX, a global federated research network. Adults with IBD and obesity (BMI ≥30) were matched 1:1 based on demographics, commodities, and baseline medication use, comparing those on GLP-1RA (Semaglutide/dulaglutide/liraglutide) or GIP/GLP-1RA (Tirzepatide) to those not on these medications. The primary composite outcome included all-cause hospitalization, steroid use, colectomy, initiation of biologic (Vedolizumab, Ustekinumab, Risankizumab), and initiation of small molecules (Upadacitinib, tofacitinib) over 10 years. Secondary outcomes included each component individually, all-cause mortality, elevated CRP≥ 10 mg/L, fecal calprotectin ≥ 50 ug/g, acute pancreatitis, and ED visits. Subgroup analysis with just Crohn’s disease (CD) and ulcerative colitis (UC) were done. Odds ratios (ORs) with 95% confidence intervals (CIs) and p-values were reported.
Results: In the matched IBD cohort (n=5,772 per group), GLP-1RA or GIP/GLP-1RA use was associated with significantly reduced risks of primary composite outcome (51% vs. 65%, OR 0.562, CI 0.522-0.606; p< 0.001), risk of hospitalization (27% vs. 43.2%), all-cause mortality (2.8% vs.12.1%), steroid use (41.2% vs 49.9%), colectomy (0.4% vs. 0.9%), ED visits (35.9% vs. 45.4%). CRP (13.7% vs. 24.1%) and fecal calprotectin (5.5% vs. 7%) elevations were higher in the non-GLP-1 RA or GIP/GLP-1 RA group. However, biologic use was more in the GLP-1RA or GIP/GLP-1 RA group (2.5% vs.1.7%; OR 1.477; p=0.003). Rates of acute pancreatitis were similar(p=0.92). Subgroup analysis in CD and UC showed consistent findings, with reduced composite outcome risk in both. No significant difference in biologic use was seen in subgroups.
Discussion: GLP-1 RA or GIP/GLP-1 RA use in IBD patients with obesity is associated with significantly improved clinical outcomes such as hospitalization, mortality, steroid use and inflammatory burden. These findings highlight a potential therapeutic benefit in modifying disease course in obese IBD patients and warrant further prospective studies.

Figure: Baseline characteristics after matching in IBD patients with obesity cohort with or without GLP-1 RA or GIP/GLP-1 RA use

Figure: Table 2: Clinical outcomes in IBD patients with obesity with vs. without GLP-1 RA or GIP/GLP-1 RA use. Subgroup analysis with UC patients with obesity and CD patients with obesity with vs. without GLP-1 RA or GIP/GLP-1 RA use
Disclosures:
Mohamed Nadeem indicated no relevant financial relationships.
Akash Khurana indicated no relevant financial relationships.
Stephen Firkins indicated no relevant financial relationships.
Roberto Simons-Linares indicated no relevant financial relationships.
Mohamed Nadeem, MD1, Akash Khurana, MD2, Stephen Firkins, MD2, Roberto Simons-Linares, MD2. P5332 - Impact of GLP-1 Receptor Agonist or GIP/GLP-1 Receptor Agonist on Clinical Outcomes in Obese Patients With Inflammatory Bowel Disease: A Propensity-Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
