Tuesday Poster Session
Category: IBD
P5412 - Gastrointestinal Adverse Effects of Sphingosine-1-Phosphate (S1P) Receptor Modulators: A Real-World Pharmacovigilance Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Pratima Jasti, MBBS (she/her/hers)
All India Institute of Medical Sciences, Bhubaneswar
Bhubaneswar, Orissa, India
Presenting Author(s)
Pratima Jasti, MBBS1, Farheen F.. Vhora, MBBS2, Ratnadeep Biswas, MBBS3, Abhay A.. Kapoor, MBBS4, Jaahnavee Trivedi, MBBS5
1All India Institute of Medical Sciences, Bhubaneswar, Bhubaneswar, Orissa, India; 2B.J. Medical College, Ahmedabad, Ahmedabad, Gujarat, India; 3ESIC Medical College & Hospital, Bihta, Patna, Patna, Bihar, India; 4St. Mary Medical Center, Langhorne, Langhorne, PA; 5SUNY Downstate Health Sciences University, Brooklyn, NY
Introduction: Sphingosine-1-phosphate (S1P) receptor modulators are increasingly used for autoimmune diseases such as multiple sclerosis and ulcerative colitis. Although effective, their frequently reported GI adverse events (AEs) may impair adherence and quality of life. However, comparative real-world data on GI AEs across different S1P modulators remain limited.
Methods: We queried the FDA Adverse Event Reporting System (FAERS) database for AEs reported for fingolimod, siponimod, ozanimod, and ponesimod for all indications from 2021-25. We determined the proportion of GI AEs and classified them by seriousness along with subsite categorization. Odds ratios (ORs) were calculated to compare GI AEs between the four drugs.
Results: Out of 26,928 S1P-related reports, 3,535 (13.1%) involved GI AEs. The proportion of GI AEs was highest for ozanimod (17.78%), followed by siponimod (13.02%), fingolimod (10.84%), and ponesimod (9.06%). Serious GI AEs accounted for 58% of cases. Fingolimod had the highest proportion of serious cases (75.3%), while ozanimod had the lowest (35.3%).
Compared to fingolimod, siponimod showed significantly lower odds of serious GI AEs (OR 0.53; 95% CI 0.44–0.64). Siponimod also had significantly reduced odds of life-threatening GI events (OR 0.54), hepatic AEs (OR 0.62), pancreatic AEs (OR 0.27), GI hemorrhage (OR 0.20), general GI AEs (OR 0.44), and oral AEs (OR 0.66).
Ozanimod also demonstrated markedly lower odds of serious GI AEs (OR 0.18; 95% CI 0.15–0.21). It was also associated with lower life-threatening GI events (OR 0.43) and hospitalization (OR 0.63). Ozanimod had lower odds of upper GI AEs (OR 0.39) but higher odds of lower GI AEs (OR 1.30). It also showed reduced odds of abdominal pain (OR 0.72), hepatic AEs (OR 0.11), pancreatic AEs (OR 0.37), GI hemorrhage (OR 0.17), general GI (OR 0.57), and oral AEs (OR 0.35).
Discussion: GI adverse effects are a notable concern with S1P modulators, varying in frequency and severity across agents. Although ozanimod yields the highest absolute proportion of GI AEs, these tend to be less severe, exhibiting a more favorable serious event profile compared to fingolimod. Siponimod’s serious GI risk is intermediate, whereas fingolimod carries the highest burden of serious and fatal GI outcomes. Clinicians should consider individual GI risk profiles, especially in patients with preexisting GI vulnerabilities, when selecting S1P modulators and monitor symptoms to optimize adherence and outcomes.
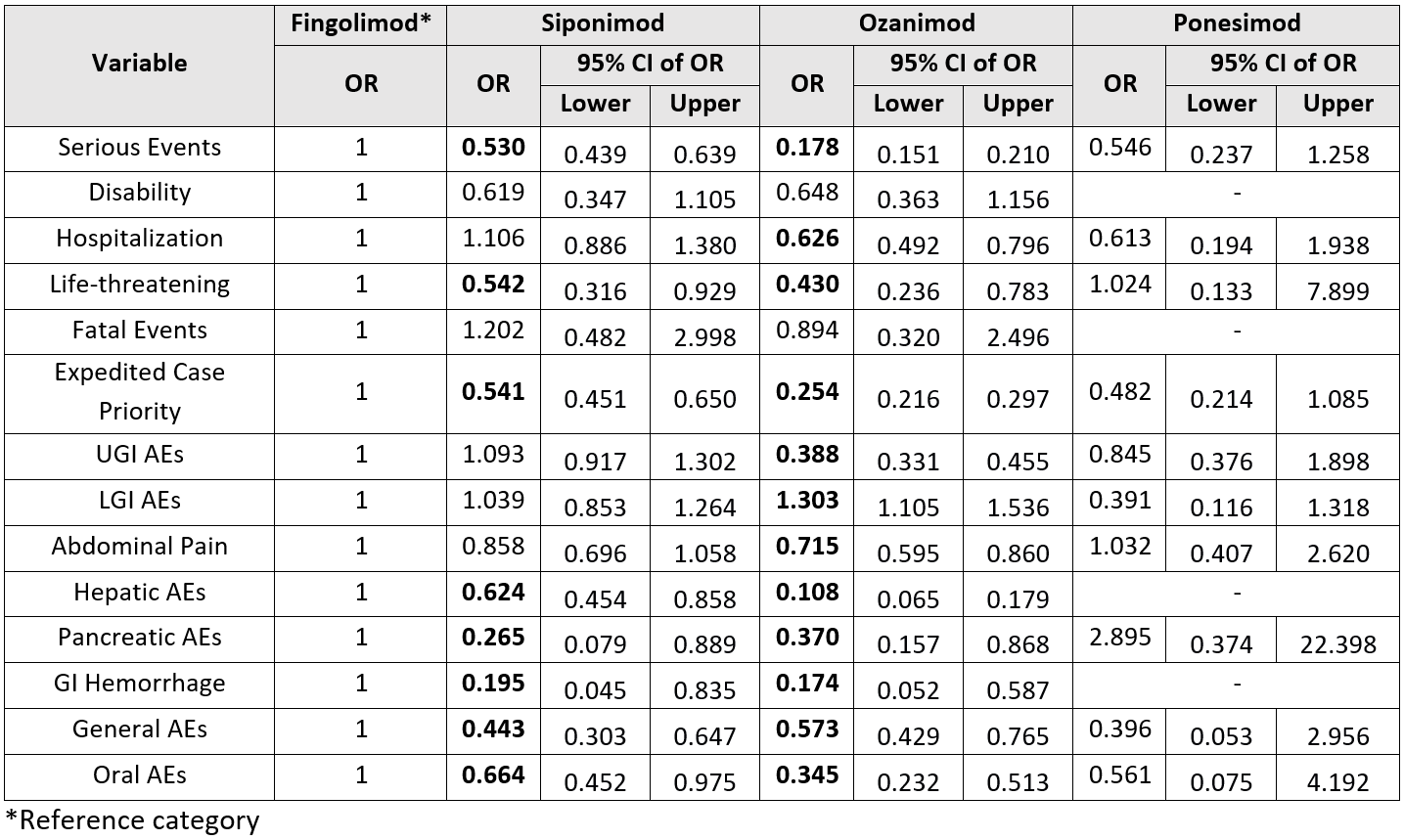
Figure: Distribution of gastrointestinal adverse events by subcategory among four S1P receptor modulators
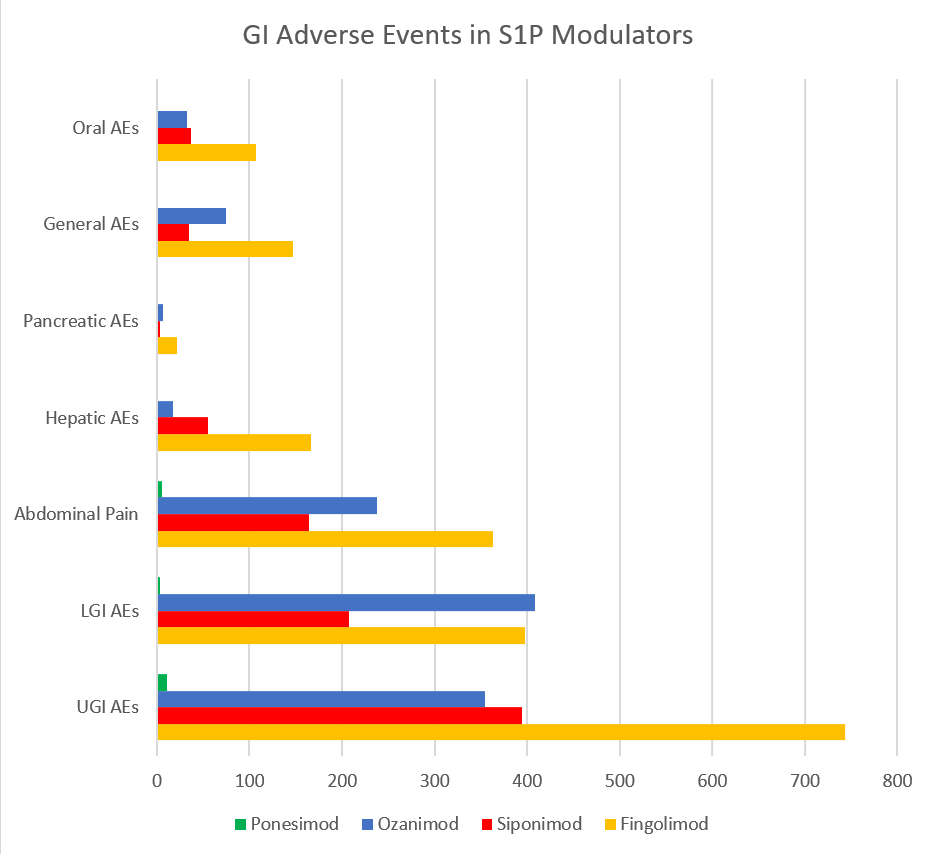
Figure: Table showing comparison of gastrointestinal adverse events between S1P receptor modulators
Disclosures:
Pratima Jasti indicated no relevant financial relationships.
Farheen Vhora indicated no relevant financial relationships.
Ratnadeep Biswas indicated no relevant financial relationships.
Abhay Kapoor indicated no relevant financial relationships.
Jaahnavee Trivedi indicated no relevant financial relationships.
Pratima Jasti, MBBS1, Farheen F.. Vhora, MBBS2, Ratnadeep Biswas, MBBS3, Abhay A.. Kapoor, MBBS4, Jaahnavee Trivedi, MBBS5. P5412 - Gastrointestinal Adverse Effects of Sphingosine-1-Phosphate (S1P) Receptor Modulators: A Real-World Pharmacovigilance Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1All India Institute of Medical Sciences, Bhubaneswar, Bhubaneswar, Orissa, India; 2B.J. Medical College, Ahmedabad, Ahmedabad, Gujarat, India; 3ESIC Medical College & Hospital, Bihta, Patna, Patna, Bihar, India; 4St. Mary Medical Center, Langhorne, Langhorne, PA; 5SUNY Downstate Health Sciences University, Brooklyn, NY
Introduction: Sphingosine-1-phosphate (S1P) receptor modulators are increasingly used for autoimmune diseases such as multiple sclerosis and ulcerative colitis. Although effective, their frequently reported GI adverse events (AEs) may impair adherence and quality of life. However, comparative real-world data on GI AEs across different S1P modulators remain limited.
Methods: We queried the FDA Adverse Event Reporting System (FAERS) database for AEs reported for fingolimod, siponimod, ozanimod, and ponesimod for all indications from 2021-25. We determined the proportion of GI AEs and classified them by seriousness along with subsite categorization. Odds ratios (ORs) were calculated to compare GI AEs between the four drugs.
Results: Out of 26,928 S1P-related reports, 3,535 (13.1%) involved GI AEs. The proportion of GI AEs was highest for ozanimod (17.78%), followed by siponimod (13.02%), fingolimod (10.84%), and ponesimod (9.06%). Serious GI AEs accounted for 58% of cases. Fingolimod had the highest proportion of serious cases (75.3%), while ozanimod had the lowest (35.3%).
Compared to fingolimod, siponimod showed significantly lower odds of serious GI AEs (OR 0.53; 95% CI 0.44–0.64). Siponimod also had significantly reduced odds of life-threatening GI events (OR 0.54), hepatic AEs (OR 0.62), pancreatic AEs (OR 0.27), GI hemorrhage (OR 0.20), general GI AEs (OR 0.44), and oral AEs (OR 0.66).
Ozanimod also demonstrated markedly lower odds of serious GI AEs (OR 0.18; 95% CI 0.15–0.21). It was also associated with lower life-threatening GI events (OR 0.43) and hospitalization (OR 0.63). Ozanimod had lower odds of upper GI AEs (OR 0.39) but higher odds of lower GI AEs (OR 1.30). It also showed reduced odds of abdominal pain (OR 0.72), hepatic AEs (OR 0.11), pancreatic AEs (OR 0.37), GI hemorrhage (OR 0.17), general GI (OR 0.57), and oral AEs (OR 0.35).
Discussion: GI adverse effects are a notable concern with S1P modulators, varying in frequency and severity across agents. Although ozanimod yields the highest absolute proportion of GI AEs, these tend to be less severe, exhibiting a more favorable serious event profile compared to fingolimod. Siponimod’s serious GI risk is intermediate, whereas fingolimod carries the highest burden of serious and fatal GI outcomes. Clinicians should consider individual GI risk profiles, especially in patients with preexisting GI vulnerabilities, when selecting S1P modulators and monitor symptoms to optimize adherence and outcomes.

Figure: Distribution of gastrointestinal adverse events by subcategory among four S1P receptor modulators

Figure: Table showing comparison of gastrointestinal adverse events between S1P receptor modulators
Disclosures:
Pratima Jasti indicated no relevant financial relationships.
Farheen Vhora indicated no relevant financial relationships.
Ratnadeep Biswas indicated no relevant financial relationships.
Abhay Kapoor indicated no relevant financial relationships.
Jaahnavee Trivedi indicated no relevant financial relationships.
Pratima Jasti, MBBS1, Farheen F.. Vhora, MBBS2, Ratnadeep Biswas, MBBS3, Abhay A.. Kapoor, MBBS4, Jaahnavee Trivedi, MBBS5. P5412 - Gastrointestinal Adverse Effects of Sphingosine-1-Phosphate (S1P) Receptor Modulators: A Real-World Pharmacovigilance Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
