Tuesday Poster Session
Category: IBD
Fidaxomicin Treatment for <i>Clostridioides difficile</i> Infection in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis
P5493 - Fidaxomicin Treatment for Clostridioides difficile Infection in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Ambar Godoy Rivas, MD
Indiana University
Indianapolis, IN
Presenting Author(s)
Ambar Godoy, MD1, Daniel Guifarro, MD2, Samreen Jawaid, MD1, Fortunato S. Principe-Meneses, MD3, Leandro Sierra, MD4, Mirian Ramirez-Rojas, 5, Renato Beas, MD6, Dalton A. Norwood, MD7, Eleazar E.. Montalvan-Sanchez, MD8
1Indiana University, Indianapolis, IN; 2Cook County Health, Chicago, IL; 3Universidad Peruana de Ciencias Aplicadas (UPC), Lima, Lima, Peru; 4Department of Internal Medicine, Cleveland Clinic, Cleveland, OH; 5Ruth Lilly Medical Library, Indiana University School of Medicine, Indianapolis, IN; 6Washington University in St Louis, St. Louis, MO; 7University of Alabama at Birmingham, Birmingham, AL; 8Yale University School of Medicine, New Haven, CT
Introduction: Patients with inflammatory bowel disease (IBD) are at increased risk for Clostridioides difficile infection (CDI), which can worsen outcomes and complicate management. Fidaxomicin is recommended for CDI, but its use in patients with IBD has not been well studied. This study summarizes available evidence on its effectiveness and safety in this population.
Methods: A systematic search of PubMed, Embase, Web of Science (through May 2025), and grey literature from DDW, ASCO, and ACG identified retrospective cohort studies reporting fidaxomicin outcomes in patients with IBD. Two reviewers independently extracted data and assessed study quality using the Newcastle-Ottawa Scale. Pooled estimates were generated via random-effects models; heterogeneity was assessed with Cochran’s Q and I².
Results: Five studies met inclusion criteria, comprising a total of 4,205 patients with IBD and CDI treated with fidaxomicin. Meta-analysis showed a pooled CDI recurrence of 10.97% (95% CI: 9.32–12.73%), mortality of 6.68% (95% CI: 1.81–13.96%), and colectomy rate of 1.57% (95% CI: 1.17–2.02%). Comparative analysis found no significant differences between fidaxomicin and vancomycin in recurrence, mortality, or colectomy. Initial cure rates ranged from 60.5% to 81.8%, and sustained response at 12 weeks reached up to 82.3%. First-episode CDI cases showed lower recurrence (4% vs. 16%; P = .06) and higher sustained response (91% vs. 75%; P = .04) than recurrent cases. Patients achieving sustained CDI response were less likely to require escalation of IBD therapy (12% vs. 20%; P = .42). Colectomy occurred in up to 5% of UC patients, usually due to refractory disease rather than CDI. Two studies comparing fidaxomicin to vancomycin showed mixed recurrence results. Fidaxomicin was well tolerated, with only mild, non-limiting adverse events.
Discussion: Fidaxomicin is a highly favorable treatment option for CDI in patients with IBD, particularly for first-episode cases. It may reduce recurrence and support disease stability, with minimal adverse events or colectomy risk. Current guidelines often extrapolate CDI data from the general population, with limited IBD-specific evidence. This is the first meta-analysis to synthesize fidaxomicin outcomes in IBD. Although one study suggested a lower need for escalation of IBD therapy with fidaxomicin, this outcome was not consistently evaluated across studies. Further prospective studies are needed to guide treatment in this high-risk group.
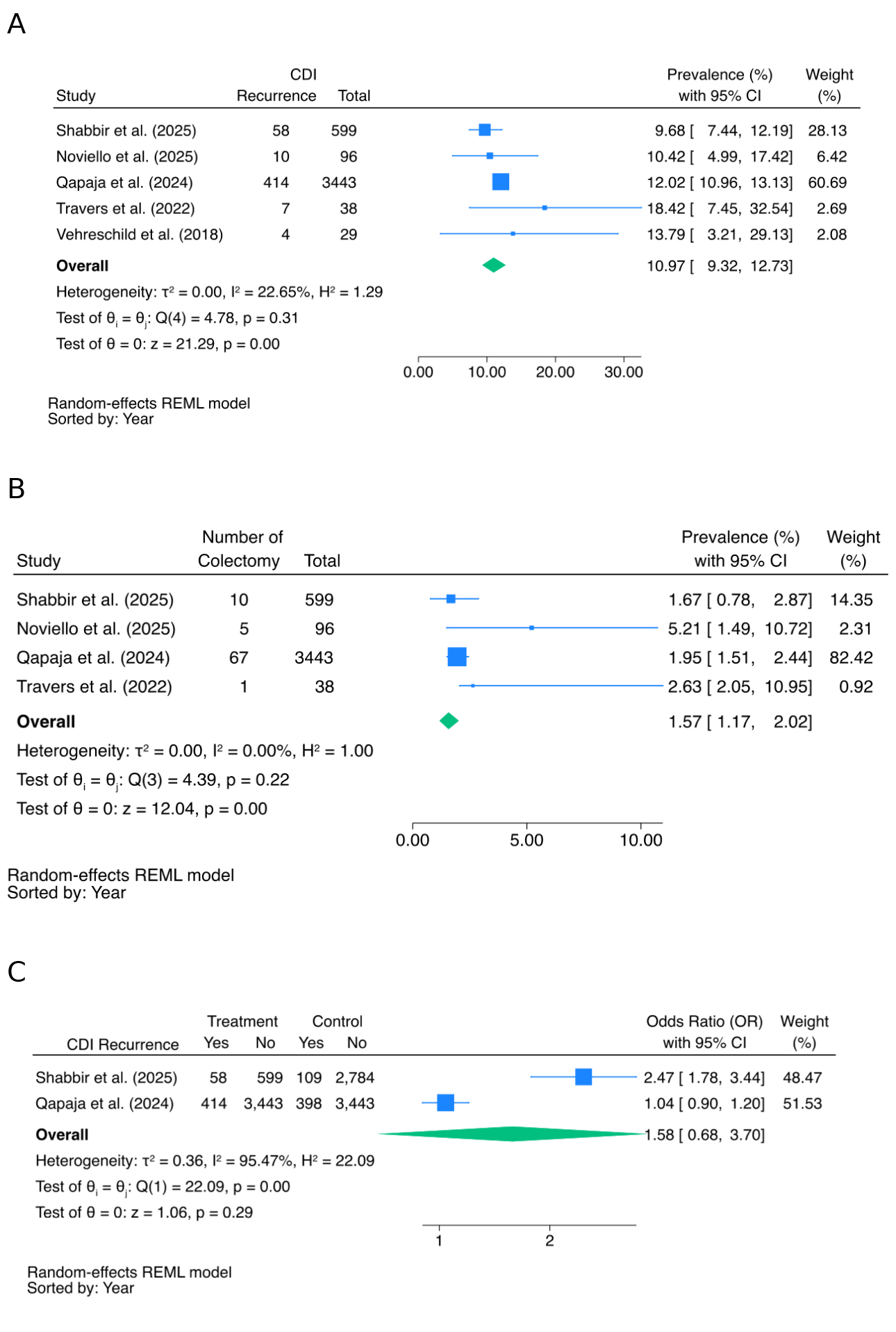
Figure: Figure 1. Forest plots summarizing outcomes of fidaxomicin treatment for Clostridioides difficile infection (CDI) in patients with inflammatory bowel disease (IBD). A. Pooled CDI recurrence rates. B. Pooled prevalence of colectomy post-fidaxomicin treatment. C. Odds ratios comparing CDI recurrence with fidaxomicin versus vancomycin.
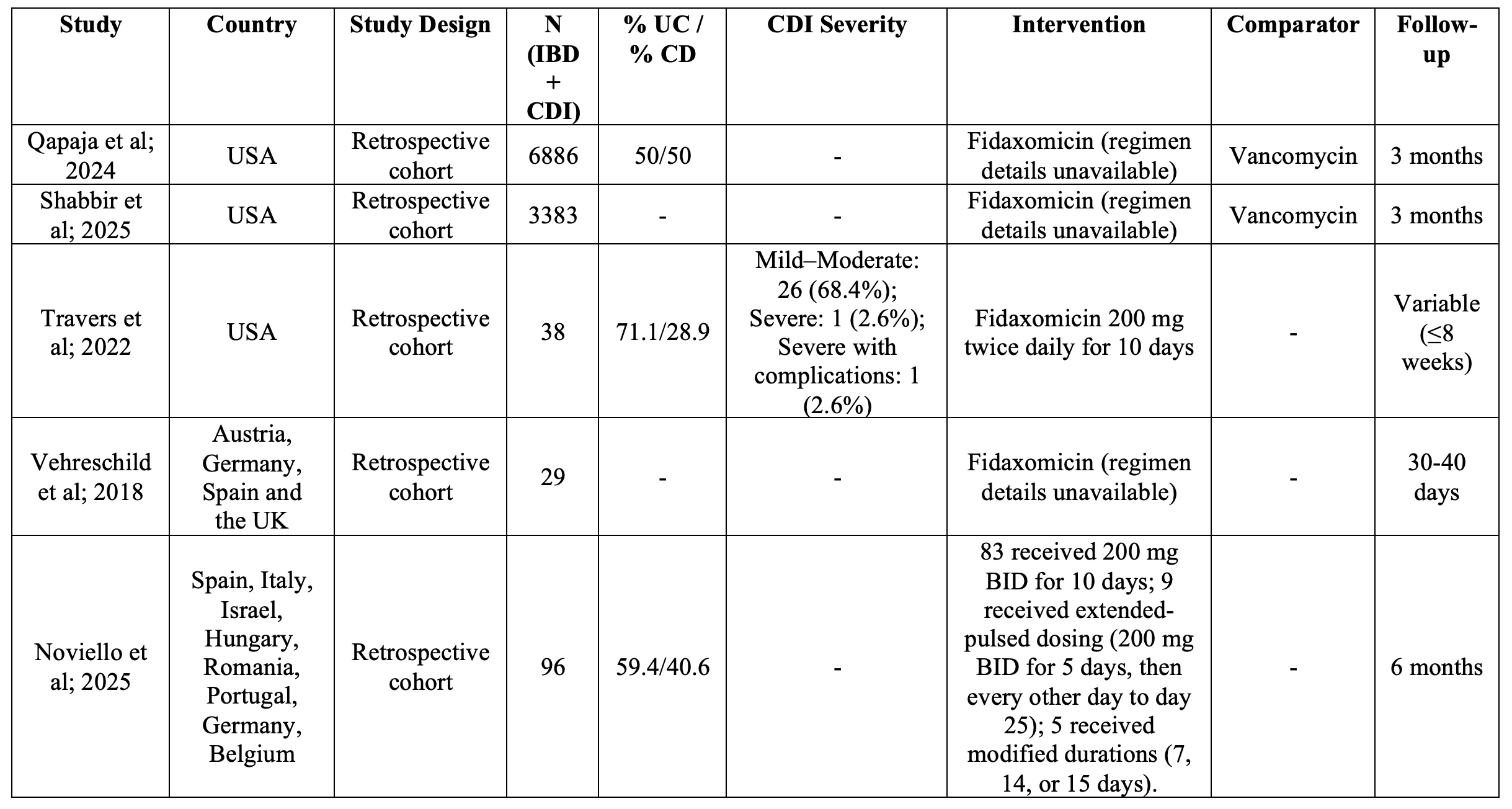
Figure: Table 1. Characteristics of included studies.
Disclosures:
Ambar Godoy indicated no relevant financial relationships.
Daniel Guifarro indicated no relevant financial relationships.
Samreen Jawaid indicated no relevant financial relationships.
Fortunato S. Principe-Meneses indicated no relevant financial relationships.
Leandro Sierra indicated no relevant financial relationships.
Mirian Ramirez-Rojas indicated no relevant financial relationships.
Renato Beas indicated no relevant financial relationships.
Dalton Norwood indicated no relevant financial relationships.
Eleazar Montalvan-Sanchez indicated no relevant financial relationships.
Ambar Godoy, MD1, Daniel Guifarro, MD2, Samreen Jawaid, MD1, Fortunato S. Principe-Meneses, MD3, Leandro Sierra, MD4, Mirian Ramirez-Rojas, 5, Renato Beas, MD6, Dalton A. Norwood, MD7, Eleazar E.. Montalvan-Sanchez, MD8. P5493 - Fidaxomicin Treatment for <i>Clostridioides difficile</i> Infection in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Indiana University, Indianapolis, IN; 2Cook County Health, Chicago, IL; 3Universidad Peruana de Ciencias Aplicadas (UPC), Lima, Lima, Peru; 4Department of Internal Medicine, Cleveland Clinic, Cleveland, OH; 5Ruth Lilly Medical Library, Indiana University School of Medicine, Indianapolis, IN; 6Washington University in St Louis, St. Louis, MO; 7University of Alabama at Birmingham, Birmingham, AL; 8Yale University School of Medicine, New Haven, CT
Introduction: Patients with inflammatory bowel disease (IBD) are at increased risk for Clostridioides difficile infection (CDI), which can worsen outcomes and complicate management. Fidaxomicin is recommended for CDI, but its use in patients with IBD has not been well studied. This study summarizes available evidence on its effectiveness and safety in this population.
Methods: A systematic search of PubMed, Embase, Web of Science (through May 2025), and grey literature from DDW, ASCO, and ACG identified retrospective cohort studies reporting fidaxomicin outcomes in patients with IBD. Two reviewers independently extracted data and assessed study quality using the Newcastle-Ottawa Scale. Pooled estimates were generated via random-effects models; heterogeneity was assessed with Cochran’s Q and I².
Results: Five studies met inclusion criteria, comprising a total of 4,205 patients with IBD and CDI treated with fidaxomicin. Meta-analysis showed a pooled CDI recurrence of 10.97% (95% CI: 9.32–12.73%), mortality of 6.68% (95% CI: 1.81–13.96%), and colectomy rate of 1.57% (95% CI: 1.17–2.02%). Comparative analysis found no significant differences between fidaxomicin and vancomycin in recurrence, mortality, or colectomy. Initial cure rates ranged from 60.5% to 81.8%, and sustained response at 12 weeks reached up to 82.3%. First-episode CDI cases showed lower recurrence (4% vs. 16%; P = .06) and higher sustained response (91% vs. 75%; P = .04) than recurrent cases. Patients achieving sustained CDI response were less likely to require escalation of IBD therapy (12% vs. 20%; P = .42). Colectomy occurred in up to 5% of UC patients, usually due to refractory disease rather than CDI. Two studies comparing fidaxomicin to vancomycin showed mixed recurrence results. Fidaxomicin was well tolerated, with only mild, non-limiting adverse events.
Discussion: Fidaxomicin is a highly favorable treatment option for CDI in patients with IBD, particularly for first-episode cases. It may reduce recurrence and support disease stability, with minimal adverse events or colectomy risk. Current guidelines often extrapolate CDI data from the general population, with limited IBD-specific evidence. This is the first meta-analysis to synthesize fidaxomicin outcomes in IBD. Although one study suggested a lower need for escalation of IBD therapy with fidaxomicin, this outcome was not consistently evaluated across studies. Further prospective studies are needed to guide treatment in this high-risk group.

Figure: Figure 1. Forest plots summarizing outcomes of fidaxomicin treatment for Clostridioides difficile infection (CDI) in patients with inflammatory bowel disease (IBD). A. Pooled CDI recurrence rates. B. Pooled prevalence of colectomy post-fidaxomicin treatment. C. Odds ratios comparing CDI recurrence with fidaxomicin versus vancomycin.

Figure: Table 1. Characteristics of included studies.
Disclosures:
Ambar Godoy indicated no relevant financial relationships.
Daniel Guifarro indicated no relevant financial relationships.
Samreen Jawaid indicated no relevant financial relationships.
Fortunato S. Principe-Meneses indicated no relevant financial relationships.
Leandro Sierra indicated no relevant financial relationships.
Mirian Ramirez-Rojas indicated no relevant financial relationships.
Renato Beas indicated no relevant financial relationships.
Dalton Norwood indicated no relevant financial relationships.
Eleazar Montalvan-Sanchez indicated no relevant financial relationships.
Ambar Godoy, MD1, Daniel Guifarro, MD2, Samreen Jawaid, MD1, Fortunato S. Principe-Meneses, MD3, Leandro Sierra, MD4, Mirian Ramirez-Rojas, 5, Renato Beas, MD6, Dalton A. Norwood, MD7, Eleazar E.. Montalvan-Sanchez, MD8. P5493 - Fidaxomicin Treatment for <i>Clostridioides difficile</i> Infection in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

