Tuesday Poster Session
Category: IBD
P5490 - Efficacy of Advanced Therapies for Naïve and Experienced Patients for Moderately-to-Severely Active Ulcerative Colitis: A Bayesian Network Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- KW
Karolina Wosik, MSc, PhD
Pfizer Canada
Kirkland, QC, Canada
Presenting Author(s)
Vipul Jairath, MBChB, DPhil, MRCP1, Thomas P. Leahy, PhD2, Ravi Potluri, PGDM3, Karolina Wosik, MSc, PhD4, David Gruben, PhD5, Joseph C. Cappelleri, PhD, MPH5, Ernest Law, PharmD6, Peter Hur, PharmD6, Tim Raine, PhD, MBBChir7
1Department of Medicine and Department of Epidemiology and Biostatistics, Western University, London, ON, Canada; 2Putnam Associates, LLC, Toronto, ON, Canada; 3Putnam Associates, LLC, New York, NY; 4Pfizer Canada, Kirkland, PQ, Canada; 5Pfizer Inc, Groton, CT; 6Pfizer Inc, New York, NY; 7Department of Gastroenterology, Cambridge University Hospitals, Cambridge, England, United Kingdom
Introduction: The evolving advanced treatment landscape for moderately-to-severely active ulcerative colitis (UC) warrants an updated efficacy assessment of a 2022 network meta-analysis (NMA)1 that included etrasimod as the newest approved agent. Since then, additional therapies including mirikizumab (MIR), risankizumab (RZB), and guselkumab (GUS) have been approved.
Methods: A systematic literature review up to 10/22/2024 was performed. A Bayesian, multinomial, fixed-effect model was used to model clinical remission and response at the end of induction and among induction phase responders at the end of maintenance, stratified by prior exposure to advanced therapies (AT-naïve [AT-N] vs AT-experienced [AT-E]). Results are reported as median relative risk (RR) with 95% credible intervals (CrI) of treatment vs comparator.
Results: Four additional studies each for induction and maintenance were included. Results of pairwise comparisons across agents from the 2022 NMA were consistent. For additional agents, most pairwise comparisons had no significant difference at the end of induction and maintenance (Figs 1 & 2).
For AT-N induction clinical remission, MIR 300mg was significantly less effective vs RZB 1200mg (RR, 0.7; 95% CrI, 0.5-1.0) and GUS 200mg was favored vs MIR 300mg (1.6, 1.1-1.2). Adalimumab (ADA) and filgotinib had significantly lower likelihoods of remission vs RZB and GUS. For AT-E induction, ADA 160/80mg (0.3, 0.2-0.7) and ozanimod 1mg (0.5, 0.2-1.0) were less favorable vs GUS.
For AT-N maintenance clinical remission, tofacitinib (TOF) 10mg and upadacitinib (UPA) 30mg were significantly more effective vs infliximab 120mg SC (TOF: 1.3, 1.0-1.9; UPA: 1.3, 1.0-2.0), MIR 200mg (TOF: 1.3, 1.0-1.9; UPA: 1.3, 1.0-2.0), and RZB 180mg (TOF: 1.7, 1.1-3.3; UPA: 1.7, 1.1-3.5) and RZB 360mg (TOF: 1.5, 1.0-2.7; UPA: 1.5, 1.0-2.9). For AT-E patients, most treatments were more effective vs RZB; RZB 360mg was not significant vs PBO (0.8, 0.6-1.0). Clinical response findings were similar for induction and maintenance.
Discussion: Pairwise comparisons of the updated NMA were consistent with the 2022 NMA and most new comparisons demonstrated similar efficacy. Differences for new agents included that RZB and GUS were favorable over MIR, ADA and FIL for AT-N induction, and RZB was less favorable vs most agents for AT-E maintenance. Future head-to-head and real-world studies should be assessed to clarify these findings.
1. Jairath et al. J Comp Eff Res. 2025;e240225
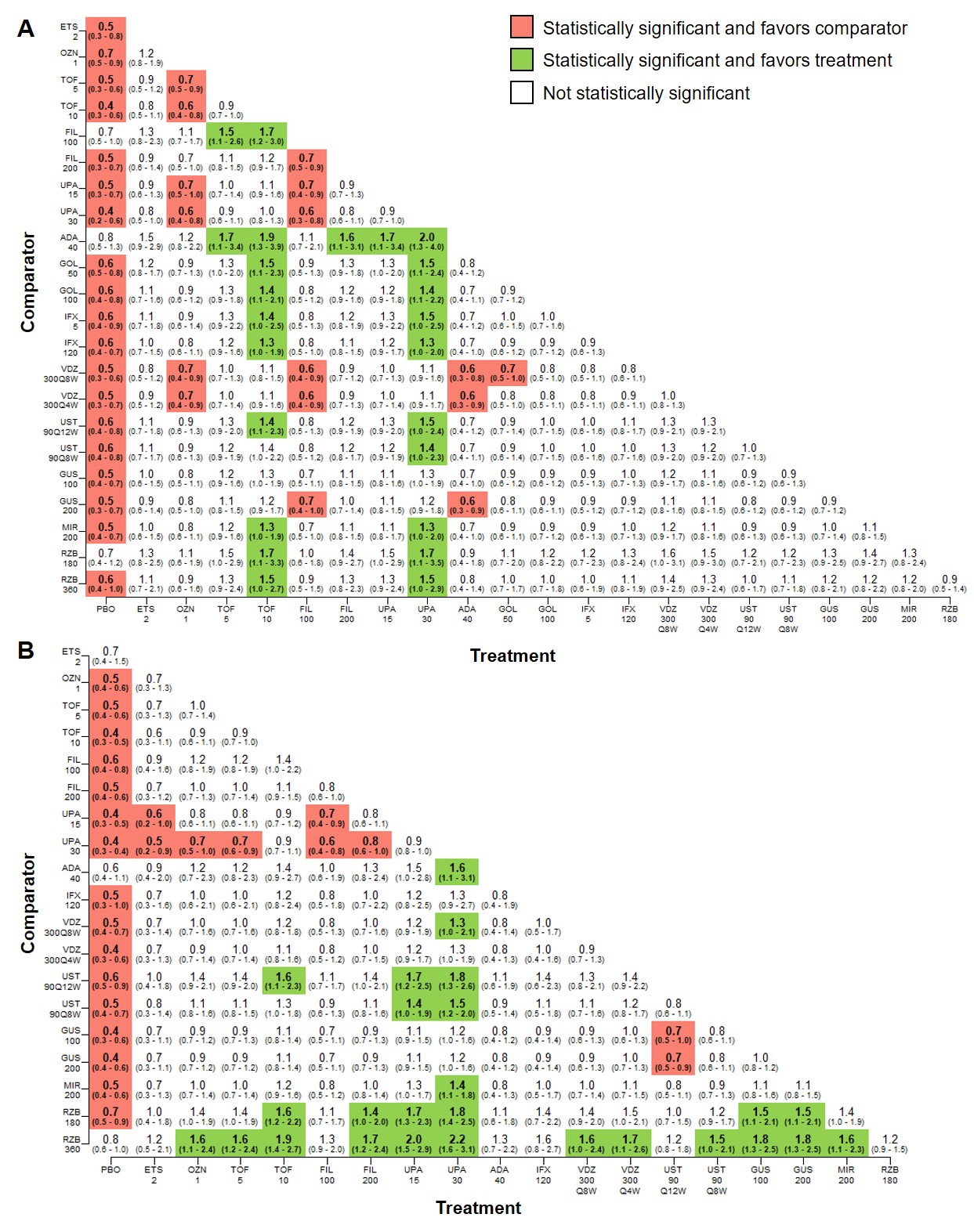
Figure: Figure 1. Pairwise comparisons among all treatments for clinical remission at the end of the induction phase for A. advanced treatment-naïve patients and B. advanced treatment-experienced patients (median RR, 95% CrI).
ADA, adalimumab; CrI, credible interval; ETS, etrasimod; FIL, filgotinib; GOL, golimumab; GUS, guselkumab; IFX, infliximab; MIR, mirikizumab; OZN, ozanimod; PBO, placebo; RR, relative risk; RZB, risankizumab; TOF, tofacitinib; UPA, upadacitinib; UST, ustekinumab; VDZ, vedolizumab.
All numbers shown on axes represent milligrams. Significance level, α=0.05.
*Weeks 0, 2, and 6 only.
**Weeks 0 and 2 only.
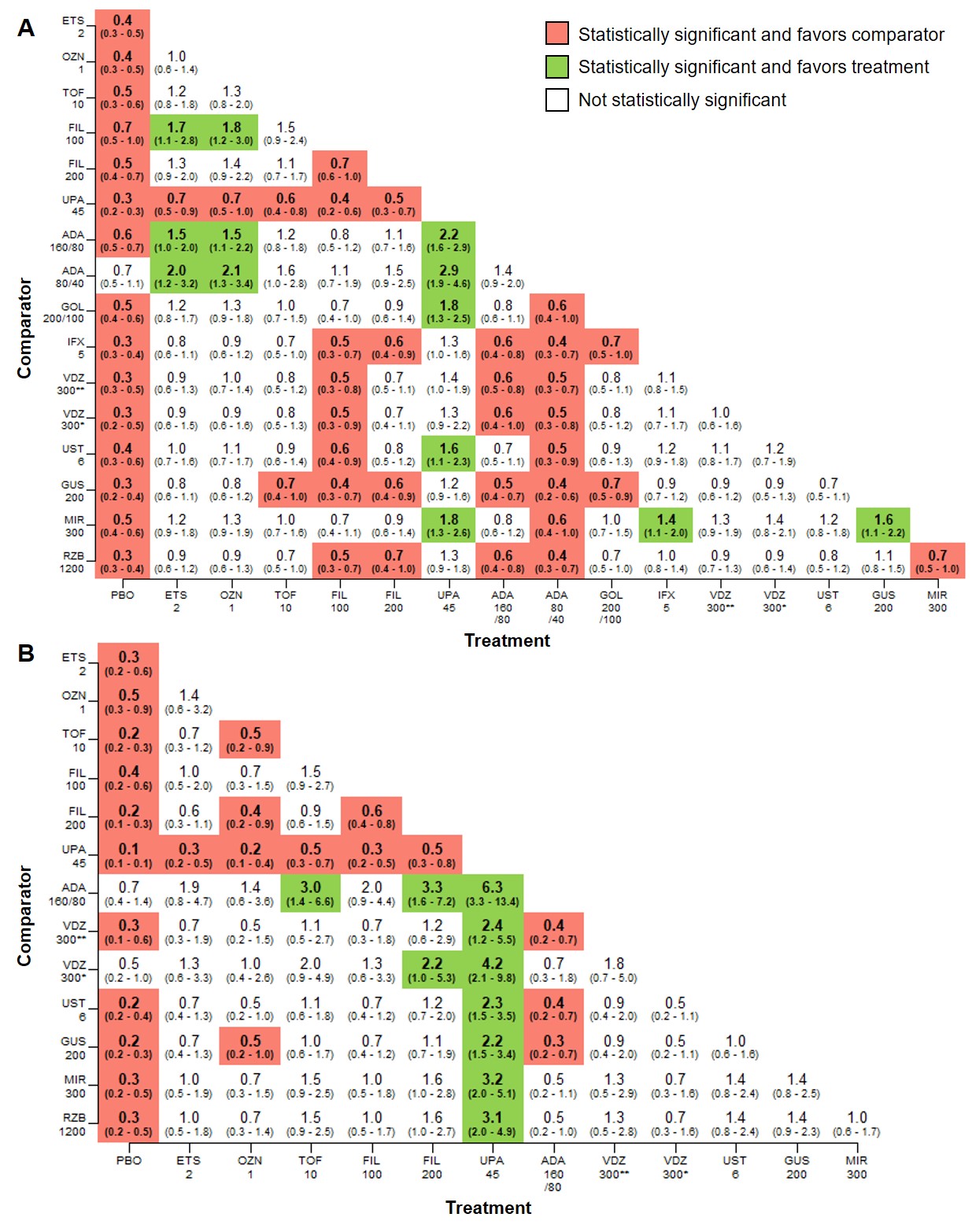
Figure: Figure 2. Pairwise comparisons among all treatments for clinical remission at the end of the maintenance phase for A. advanced treatment-naïve patients and B. advanced treatment-experienced patients (median RR, 95% CrI).
ADA, adalimumab; CrI, credible interval; ETS, etrasimod; FIL, filgotinib; GOL, golimumab; GUS, guselkumab; IFX, infliximab; MIR, mirikizumab; OZN, ozanimod; PBO, placebo; Q4W, every 4 weeks; Q8W, every 8 weeks; Q12W, every 12 weeks; RR, relative risk; RZB, risankizumab; TOF, tofacitinib; UPA, upadacitinib; UST, ustekinumab; VDZ, vedolizumab.
All numbers shown on axes represent milligrams. Significance level, α=0.05.
Disclosures:
Vipul Jairath: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Pharma – Consultant, Grant/Research Support, Speakers Bureau. Asieris Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. Avoro Capital – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Endpoint Health – Consultant. Enthera – Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Flagship Pioneering – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Grant/Research Support, Speakers Bureau. Galapagos NV – Consultant, Grant/Research Support, Speakers Bureau. Genentech – Consultant, Grant/Research Support, Speakers Bureau. Gilde Healthcare – Consultant. Gilead Sciences – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Grant/Research Support, Speakers Bureau. Innomar – Consultant. JAMP – Consultant. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Merck – Consultant, Grant/Research Support, Speakers Bureau. Metacrine – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Pandion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Pendopharm – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Therapeutics and Diagnostics – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Reistone Biopharma – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Roivant – Consultant. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. Second Genome – Consultant, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Sorriso Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Syndegen – Consultant. Takeda – Consultant, Intellectual Property/Patents, Speakers Bureau. TD Securities – Consultant. Teva – Consultant, Grant/Research Support, Speakers Bureau. Topivert – Consultant, Grant/Research Support, Speakers Bureau. Ventyx Biosciences – Consultant, Grant/Research Support, Speakers Bureau. Vividion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Thomas Leahy: Pfizer Inc – Stock Options, Stock-publicly held company(excluding mutual/index funds). Putnam Associates – Employee.
Ravi Potluri: Pfizer Inc – Stock Options, Stock-publicly held company(excluding mutual/index funds). Putnam Associates – Employee.
Karolina Wosik: Pfizer Canada Inc – Employee. Pfizer Inc – Stock Options.
David Gruben: Pfizer Inc – Employee, Stock Options.
Joseph Cappelleri: Pfizer Inc – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Ernest Law: Pfizer Inc – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Peter Hur: AbbVie – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Buhlmann – Grant/Research Support. Clene Nanomedicine – Stock Options. Haleon – Stock Options. Idorsia – Stock Options. Janssen – Grant/Research Support. Lilly – Grant/Research Support. Liquidia – Stock Options. Longboard Pharmaceuticals – Stock Options. Pfizer Inc – Employee, Grant/Research Support, Stock Options. Proctor & Gamble – Stock Options. Takeda – Grant/Research Support. US 2022/0257594 A1 – Intellectual Property/Patents.
Tim Raine: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alfasigma – Consultant, Grant/Research Support, Speakers Bureau. Arena – Consultant, Grant/Research Support, Speakers Bureau. Aslan – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. BMS – Consultant, Grant/Research Support, Speakers Bureau. Boehringer-Ingelheim – Consultant, Grant/Research Support, Speakers Bureau. Celgene – Consultant, Grant/Research Support, Speakers Bureau. Domain Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly – Consultant, Grant/Research Support, Speakers Bureau. Ferring – Consultant, Grant/Research Support, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Gilead – Consultant, Grant/Research Support, Speakers Bureau. GSK – Consultant, Grant/Research Support, Speakers Bureau. Heptares – Consultant, Grant/Research Support, Speakers Bureau. Janssen – Consultant, Grant/Research Support, Speakers Bureau. LabGenius – Consultant, Grant/Research Support, Speakers Bureau. MonteRosa – Consultant, Grant/Research Support, Speakers Bureau. MSD – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Novartis – Consultant, Speakers Bureau. Numab – Consultant, Grant/Research Support, Speakers Bureau. Pfizer Inc – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. Scientia – Consultant, Grant/Research Support, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. UCB – Consultant, Grant/Research Support, Speakers Bureau. XAP therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Vipul Jairath, MBChB, DPhil, MRCP1, Thomas P. Leahy, PhD2, Ravi Potluri, PGDM3, Karolina Wosik, MSc, PhD4, David Gruben, PhD5, Joseph C. Cappelleri, PhD, MPH5, Ernest Law, PharmD6, Peter Hur, PharmD6, Tim Raine, PhD, MBBChir7. P5490 - Efficacy of Advanced Therapies for Naïve and Experienced Patients for Moderately-to-Severely Active Ulcerative Colitis: A Bayesian Network Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Medicine and Department of Epidemiology and Biostatistics, Western University, London, ON, Canada; 2Putnam Associates, LLC, Toronto, ON, Canada; 3Putnam Associates, LLC, New York, NY; 4Pfizer Canada, Kirkland, PQ, Canada; 5Pfizer Inc, Groton, CT; 6Pfizer Inc, New York, NY; 7Department of Gastroenterology, Cambridge University Hospitals, Cambridge, England, United Kingdom
Introduction: The evolving advanced treatment landscape for moderately-to-severely active ulcerative colitis (UC) warrants an updated efficacy assessment of a 2022 network meta-analysis (NMA)1 that included etrasimod as the newest approved agent. Since then, additional therapies including mirikizumab (MIR), risankizumab (RZB), and guselkumab (GUS) have been approved.
Methods: A systematic literature review up to 10/22/2024 was performed. A Bayesian, multinomial, fixed-effect model was used to model clinical remission and response at the end of induction and among induction phase responders at the end of maintenance, stratified by prior exposure to advanced therapies (AT-naïve [AT-N] vs AT-experienced [AT-E]). Results are reported as median relative risk (RR) with 95% credible intervals (CrI) of treatment vs comparator.
Results: Four additional studies each for induction and maintenance were included. Results of pairwise comparisons across agents from the 2022 NMA were consistent. For additional agents, most pairwise comparisons had no significant difference at the end of induction and maintenance (Figs 1 & 2).
For AT-N induction clinical remission, MIR 300mg was significantly less effective vs RZB 1200mg (RR, 0.7; 95% CrI, 0.5-1.0) and GUS 200mg was favored vs MIR 300mg (1.6, 1.1-1.2). Adalimumab (ADA) and filgotinib had significantly lower likelihoods of remission vs RZB and GUS. For AT-E induction, ADA 160/80mg (0.3, 0.2-0.7) and ozanimod 1mg (0.5, 0.2-1.0) were less favorable vs GUS.
For AT-N maintenance clinical remission, tofacitinib (TOF) 10mg and upadacitinib (UPA) 30mg were significantly more effective vs infliximab 120mg SC (TOF: 1.3, 1.0-1.9; UPA: 1.3, 1.0-2.0), MIR 200mg (TOF: 1.3, 1.0-1.9; UPA: 1.3, 1.0-2.0), and RZB 180mg (TOF: 1.7, 1.1-3.3; UPA: 1.7, 1.1-3.5) and RZB 360mg (TOF: 1.5, 1.0-2.7; UPA: 1.5, 1.0-2.9). For AT-E patients, most treatments were more effective vs RZB; RZB 360mg was not significant vs PBO (0.8, 0.6-1.0). Clinical response findings were similar for induction and maintenance.
Discussion: Pairwise comparisons of the updated NMA were consistent with the 2022 NMA and most new comparisons demonstrated similar efficacy. Differences for new agents included that RZB and GUS were favorable over MIR, ADA and FIL for AT-N induction, and RZB was less favorable vs most agents for AT-E maintenance. Future head-to-head and real-world studies should be assessed to clarify these findings.
1. Jairath et al. J Comp Eff Res. 2025;e240225

Figure: Figure 1. Pairwise comparisons among all treatments for clinical remission at the end of the induction phase for A. advanced treatment-naïve patients and B. advanced treatment-experienced patients (median RR, 95% CrI).
ADA, adalimumab; CrI, credible interval; ETS, etrasimod; FIL, filgotinib; GOL, golimumab; GUS, guselkumab; IFX, infliximab; MIR, mirikizumab; OZN, ozanimod; PBO, placebo; RR, relative risk; RZB, risankizumab; TOF, tofacitinib; UPA, upadacitinib; UST, ustekinumab; VDZ, vedolizumab.
All numbers shown on axes represent milligrams. Significance level, α=0.05.
*Weeks 0, 2, and 6 only.
**Weeks 0 and 2 only.

Figure: Figure 2. Pairwise comparisons among all treatments for clinical remission at the end of the maintenance phase for A. advanced treatment-naïve patients and B. advanced treatment-experienced patients (median RR, 95% CrI).
ADA, adalimumab; CrI, credible interval; ETS, etrasimod; FIL, filgotinib; GOL, golimumab; GUS, guselkumab; IFX, infliximab; MIR, mirikizumab; OZN, ozanimod; PBO, placebo; Q4W, every 4 weeks; Q8W, every 8 weeks; Q12W, every 12 weeks; RR, relative risk; RZB, risankizumab; TOF, tofacitinib; UPA, upadacitinib; UST, ustekinumab; VDZ, vedolizumab.
All numbers shown on axes represent milligrams. Significance level, α=0.05.
Disclosures:
Vipul Jairath: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Pharma – Consultant, Grant/Research Support, Speakers Bureau. Asieris Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. Avoro Capital – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Endpoint Health – Consultant. Enthera – Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Flagship Pioneering – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Grant/Research Support, Speakers Bureau. Galapagos NV – Consultant, Grant/Research Support, Speakers Bureau. Genentech – Consultant, Grant/Research Support, Speakers Bureau. Gilde Healthcare – Consultant. Gilead Sciences – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Grant/Research Support, Speakers Bureau. Innomar – Consultant. JAMP – Consultant. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Merck – Consultant, Grant/Research Support, Speakers Bureau. Metacrine – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Pandion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Pendopharm – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Therapeutics and Diagnostics – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Reistone Biopharma – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Roivant – Consultant. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. Second Genome – Consultant, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Sorriso Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Syndegen – Consultant. Takeda – Consultant, Intellectual Property/Patents, Speakers Bureau. TD Securities – Consultant. Teva – Consultant, Grant/Research Support, Speakers Bureau. Topivert – Consultant, Grant/Research Support, Speakers Bureau. Ventyx Biosciences – Consultant, Grant/Research Support, Speakers Bureau. Vividion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Thomas Leahy: Pfizer Inc – Stock Options, Stock-publicly held company(excluding mutual/index funds). Putnam Associates – Employee.
Ravi Potluri: Pfizer Inc – Stock Options, Stock-publicly held company(excluding mutual/index funds). Putnam Associates – Employee.
Karolina Wosik: Pfizer Canada Inc – Employee. Pfizer Inc – Stock Options.
David Gruben: Pfizer Inc – Employee, Stock Options.
Joseph Cappelleri: Pfizer Inc – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Ernest Law: Pfizer Inc – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Peter Hur: AbbVie – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Buhlmann – Grant/Research Support. Clene Nanomedicine – Stock Options. Haleon – Stock Options. Idorsia – Stock Options. Janssen – Grant/Research Support. Lilly – Grant/Research Support. Liquidia – Stock Options. Longboard Pharmaceuticals – Stock Options. Pfizer Inc – Employee, Grant/Research Support, Stock Options. Proctor & Gamble – Stock Options. Takeda – Grant/Research Support. US 2022/0257594 A1 – Intellectual Property/Patents.
Tim Raine: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alfasigma – Consultant, Grant/Research Support, Speakers Bureau. Arena – Consultant, Grant/Research Support, Speakers Bureau. Aslan – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. BMS – Consultant, Grant/Research Support, Speakers Bureau. Boehringer-Ingelheim – Consultant, Grant/Research Support, Speakers Bureau. Celgene – Consultant, Grant/Research Support, Speakers Bureau. Domain Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly – Consultant, Grant/Research Support, Speakers Bureau. Ferring – Consultant, Grant/Research Support, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Gilead – Consultant, Grant/Research Support, Speakers Bureau. GSK – Consultant, Grant/Research Support, Speakers Bureau. Heptares – Consultant, Grant/Research Support, Speakers Bureau. Janssen – Consultant, Grant/Research Support, Speakers Bureau. LabGenius – Consultant, Grant/Research Support, Speakers Bureau. MonteRosa – Consultant, Grant/Research Support, Speakers Bureau. MSD – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Novartis – Consultant, Speakers Bureau. Numab – Consultant, Grant/Research Support, Speakers Bureau. Pfizer Inc – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. Scientia – Consultant, Grant/Research Support, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. UCB – Consultant, Grant/Research Support, Speakers Bureau. XAP therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Vipul Jairath, MBChB, DPhil, MRCP1, Thomas P. Leahy, PhD2, Ravi Potluri, PGDM3, Karolina Wosik, MSc, PhD4, David Gruben, PhD5, Joseph C. Cappelleri, PhD, MPH5, Ernest Law, PharmD6, Peter Hur, PharmD6, Tim Raine, PhD, MBBChir7. P5490 - Efficacy of Advanced Therapies for Naïve and Experienced Patients for Moderately-to-Severely Active Ulcerative Colitis: A Bayesian Network Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
