Tuesday Poster Session
Category: Liver
Mesenchymal Stem Cell Therapy in Liver Failure: A Time-Stratified Meta-Analysis Integrating Human Clinical Trials, <i>In Vitro</i> Studies, and Animal Models
P5877 - Mesenchymal Stem Cell Therapy in Liver Failure: A Time-Stratified Meta-Analysis Integrating Human Clinical Trials, In Vitro Studies, and Animal Models
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- MK
Meghana Kakarla, MD
Infirmary Health
Mobile, AL
Presenting Author(s)
Meghana Kakarla, MD1, Vasanthi Panivel, PhD2, Ashvath Pillai, 3, Giovannie Isaac-Coss, MD4, Nilay Bhatt, MD5, Muhammad Ali Khan, MBBS6, Uchit Thapa, MD7, Venu Ganipisetti, MD8, Kashif Ali, MD9, Srinivas Chari, PhD2
1Infirmary Health, Mobile, AL; 2V S biologics Pvt LTD, Bangalore, Karnataka, India; 3SSPM Medical College and Lifetime Hospital, Navi Mumbai, Maharashtra, India; 4Rush University Medical Center, Chicago, IL; 5HCA Houston Clear Lake, Houston, TX; 6Mayo Clinic, Phoenix, AZ; 7Baystate Medical Center, Hartford, CT; 8Baystate Medical Center, Longmeadow, MA; 9University of Texas Rio Grande Valley, Edinburg, TX
Introduction: End-stage liver disease and acute liver failure patients have limited treatment options due to organ shortages and transplant complications. Mesenchymal stem cells show promising regenerative potential. This study analyzes time-dependent MSC outcomes using clinical trials, in-vitro studies, and animal models.
Methods: The research team conducted systematic database searches across PubMed, Scopus, Web of Science, Embase, ClinicalTrials.gov, preprint servers and grey literature databases through May 2025 for all liver regenerative therapy studies including in vitro experiments and animal and clinical trials. A meta-analysis evaluated efficacy and safety of MSC therapy. A risk assessment for bias was performed while PRISMA guidelines were followed.
Results: The animal experiments showed MSCs operated through immune regulation to eliminate fibrosis while enhancing both survival times and gene therapy approaches restored liver functions in targeted situations. The scaffold-based methods provided temporary liver functionality support to rodent and primate subjects during the short-term. The research conducted with iPSC-derived liver organoids in vitro showed that Wnt signaling and IL-6/STAT3 activation acted as essential pathways for regeneration. The clinical trial meta-analysis results showed MSCs reduced MELD scores by −0.32 (95% CI: −0.55 to −0.10) during the first 4 weeks and by −0.42 (95% CI: −0.82 to −0.02) during the 12-week period and by −0.91 (95% CI: −1.20 to −0.61) during the 24-week assessment but showed no effect at 48 weeks (0.13; 95% CI: −0.48 to 0.74). The pooled effect size was SMD −0.22 (95% CI: −0.33 to −0.12; p < 0.001; I² = 68.3%). The studies showed MSCs elevated albumin levels during all assessment periods extending from 2 weeks (SMD 0.81; 95% CI: 0.50 to 1.11) up to 48 weeks (0.46; 95% CI: 0.37 to 1.30). The combined results showed SMD 0.55 (95% CI: 0.37 to 0.73; p < 0.001; I² = 78.1%). The survival assessment demonstrated statistical significance only at the 48-week point (OR 1.29; 95% CI: 1.03 to 1.60; p = 0.023; I² = 0.0%).
Discussion: This first time-stratified meta-analysis of MSC therapy in liver failure shows MELD and albumin gains (2–24 weeks) and survival benefits at 48 weeks. While generally safe, risks include immune reactions, thrombosis, and reduced efficacy over time. These findings warrant future trials with standardized protocols and adjunctive regenerative strategies to sustain therapeutic benefits.
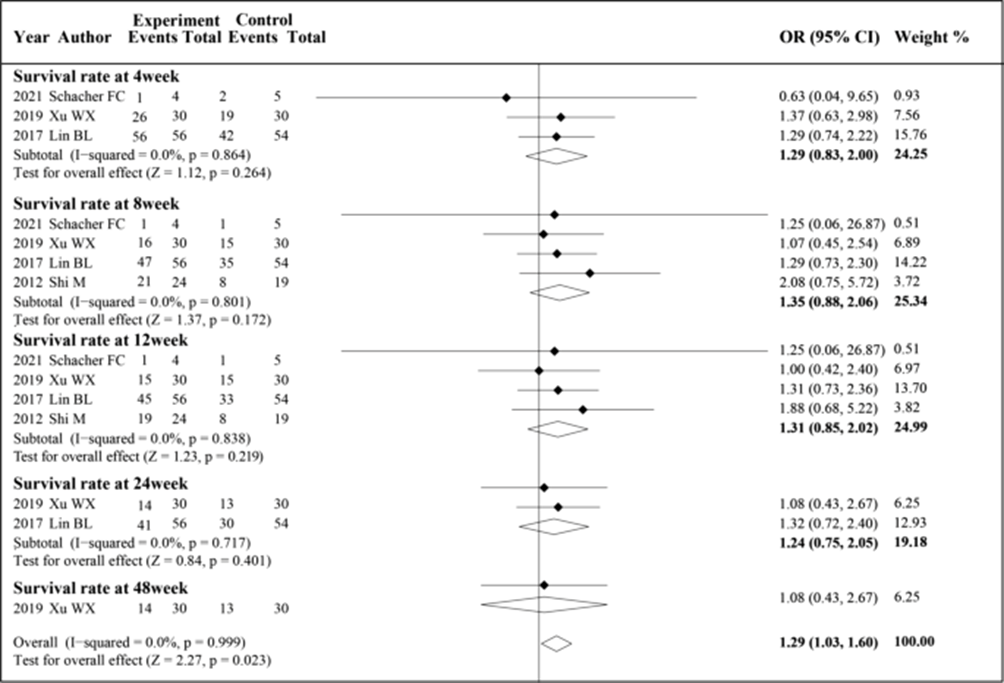
Figure: A-Changes in MELD score with Msc therapy vs standard
B-Changes in albumin with Msc therapy vs standard
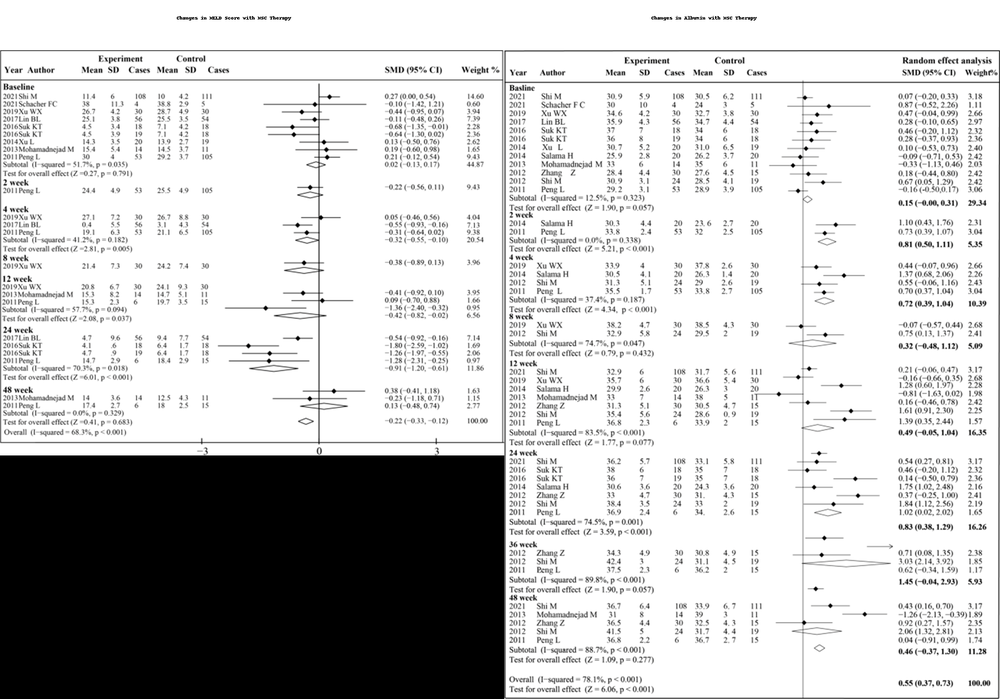
Figure: Forest plot of patient survival outcomes with MSC therapy vs standard
Disclosures:
Meghana Kakarla indicated no relevant financial relationships.
Vasanthi Panivel indicated no relevant financial relationships.
Ashvath Pillai indicated no relevant financial relationships.
Giovannie Isaac-Coss indicated no relevant financial relationships.
Nilay Bhatt indicated no relevant financial relationships.
Muhammad Ali Khan indicated no relevant financial relationships.
Uchit Thapa indicated no relevant financial relationships.
Venu Ganipisetti indicated no relevant financial relationships.
Kashif Ali indicated no relevant financial relationships.
Srinivas Chari indicated no relevant financial relationships.
Meghana Kakarla, MD1, Vasanthi Panivel, PhD2, Ashvath Pillai, 3, Giovannie Isaac-Coss, MD4, Nilay Bhatt, MD5, Muhammad Ali Khan, MBBS6, Uchit Thapa, MD7, Venu Ganipisetti, MD8, Kashif Ali, MD9, Srinivas Chari, PhD2. P5877 - Mesenchymal Stem Cell Therapy in Liver Failure: A Time-Stratified Meta-Analysis Integrating Human Clinical Trials, <i>In Vitro</i> Studies, and Animal Models, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Infirmary Health, Mobile, AL; 2V S biologics Pvt LTD, Bangalore, Karnataka, India; 3SSPM Medical College and Lifetime Hospital, Navi Mumbai, Maharashtra, India; 4Rush University Medical Center, Chicago, IL; 5HCA Houston Clear Lake, Houston, TX; 6Mayo Clinic, Phoenix, AZ; 7Baystate Medical Center, Hartford, CT; 8Baystate Medical Center, Longmeadow, MA; 9University of Texas Rio Grande Valley, Edinburg, TX
Introduction: End-stage liver disease and acute liver failure patients have limited treatment options due to organ shortages and transplant complications. Mesenchymal stem cells show promising regenerative potential. This study analyzes time-dependent MSC outcomes using clinical trials, in-vitro studies, and animal models.
Methods: The research team conducted systematic database searches across PubMed, Scopus, Web of Science, Embase, ClinicalTrials.gov, preprint servers and grey literature databases through May 2025 for all liver regenerative therapy studies including in vitro experiments and animal and clinical trials. A meta-analysis evaluated efficacy and safety of MSC therapy. A risk assessment for bias was performed while PRISMA guidelines were followed.
Results: The animal experiments showed MSCs operated through immune regulation to eliminate fibrosis while enhancing both survival times and gene therapy approaches restored liver functions in targeted situations. The scaffold-based methods provided temporary liver functionality support to rodent and primate subjects during the short-term. The research conducted with iPSC-derived liver organoids in vitro showed that Wnt signaling and IL-6/STAT3 activation acted as essential pathways for regeneration. The clinical trial meta-analysis results showed MSCs reduced MELD scores by −0.32 (95% CI: −0.55 to −0.10) during the first 4 weeks and by −0.42 (95% CI: −0.82 to −0.02) during the 12-week period and by −0.91 (95% CI: −1.20 to −0.61) during the 24-week assessment but showed no effect at 48 weeks (0.13; 95% CI: −0.48 to 0.74). The pooled effect size was SMD −0.22 (95% CI: −0.33 to −0.12; p < 0.001; I² = 68.3%). The studies showed MSCs elevated albumin levels during all assessment periods extending from 2 weeks (SMD 0.81; 95% CI: 0.50 to 1.11) up to 48 weeks (0.46; 95% CI: 0.37 to 1.30). The combined results showed SMD 0.55 (95% CI: 0.37 to 0.73; p < 0.001; I² = 78.1%). The survival assessment demonstrated statistical significance only at the 48-week point (OR 1.29; 95% CI: 1.03 to 1.60; p = 0.023; I² = 0.0%).
Discussion: This first time-stratified meta-analysis of MSC therapy in liver failure shows MELD and albumin gains (2–24 weeks) and survival benefits at 48 weeks. While generally safe, risks include immune reactions, thrombosis, and reduced efficacy over time. These findings warrant future trials with standardized protocols and adjunctive regenerative strategies to sustain therapeutic benefits.

Figure: A-Changes in MELD score with Msc therapy vs standard
B-Changes in albumin with Msc therapy vs standard

Figure: Forest plot of patient survival outcomes with MSC therapy vs standard
Disclosures:
Meghana Kakarla indicated no relevant financial relationships.
Vasanthi Panivel indicated no relevant financial relationships.
Ashvath Pillai indicated no relevant financial relationships.
Giovannie Isaac-Coss indicated no relevant financial relationships.
Nilay Bhatt indicated no relevant financial relationships.
Muhammad Ali Khan indicated no relevant financial relationships.
Uchit Thapa indicated no relevant financial relationships.
Venu Ganipisetti indicated no relevant financial relationships.
Kashif Ali indicated no relevant financial relationships.
Srinivas Chari indicated no relevant financial relationships.
Meghana Kakarla, MD1, Vasanthi Panivel, PhD2, Ashvath Pillai, 3, Giovannie Isaac-Coss, MD4, Nilay Bhatt, MD5, Muhammad Ali Khan, MBBS6, Uchit Thapa, MD7, Venu Ganipisetti, MD8, Kashif Ali, MD9, Srinivas Chari, PhD2. P5877 - Mesenchymal Stem Cell Therapy in Liver Failure: A Time-Stratified Meta-Analysis Integrating Human Clinical Trials, <i>In Vitro</i> Studies, and Animal Models, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

