Tuesday Poster Session
Category: Liver
P5930 - Evaluating the Role of Advanced Biologic Therapies on Liver Biochemistry in Concurrent Primary Sclerosing Cholangitis and Inflammatory Bowel Disease
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Benjamin L. Robinson, DO (he/him/his)
Atrium Health Carolinas Medical Center
Charlotte, NC
Presenting Author(s)
Award: ACG Presidential Poster Award
Benjamin L. Robinson, DO1, Patrick D. Green, MD2, Maithili V. Chitnavis, MD, FACG3, Andrew S. DeLemos, MD3
1Atrium Health Carolinas Medical Center, Charlotte, NC; 2Wake Forest Baptist Health, Winston-Salem, NC; 3Atrium Health, Charlotte, NC
Introduction: The overlap between primary sclerosing cholangitis (PSC) and inflammatory bowel disease (IBD) suggests a shared immunopathogenesis, raising the possibility that targeting key inflammatory pathways may modify the course of both diseases. With conventional biologics showing limited impact on PSC, this study explores the potential of novel IBD-directed therapies to influence disease trajectory in PSC-IBD.
Methods: We conducted a multi-center, retrospective cohort study of adult patients with PSC-IBD treated with upadacitinib, ustekinumab, risankizumab, or vedolizumab, using patient data between 01/2019 and 12/2024. Baseline demographic, clinical, and biochemical data were collected (Table 1). Liver biochemistries and available magnetic resonance cholangiopancreatography (MRCP) imaging were analyzed over 12 months.
Results: Data were collected from 45 patients with PSC-IBD: upadacitinib (n=10), ustekinumab (n=8), risankizumab (n=11), and vedolizumab (n=16). Across cohorts, most patients were white (75%) males (66%) with ulcerative colitis (70.6%) and a median PSC duration of 5 years (IQR 3–6.5). At baseline, 34.8% were receiving ursodeoxycholic acid, and 31.3% had cirrhosis, with a median Mayo PSC Risk Score of –0.1 (IQR –0.77 to 1.13). Large duct PSC was more common (76%) than small duct PSC, and 18% had autoimmune hepatitis (AIH) overlap. In the upadacitinib group, median ALP declined significantly from 184.5 U/L (IQR 152.2–283.2) at baseline to 125 (111.5–139.2) at 3 months, 119 (106–127.8) at 6 months, and 100.5 (92.2–115.2) at 12 months (Figure 1, p = 0.01). No other treatment groups showed significant ALP changes over time, and no significant differences in median AST, ALT, bilirubin, or platelets were observed across any groups from baseline to 12 months. The proportion of patients achieving a >20% reduction in ALP by 12 months was 80% with upadacitinib, 50% with ustekinumab, 63% with risankizumab, and 19% with vedolizumab. Among patients with follow-up MRCP at least 3 months after treatment initiation (n=23), upadacitinib was the only treatment group in which a patient showed radiographic improvement in biliary stricturing and ductal dilation compared to baseline.
Discussion: Upadacitinib was associated with significant ALP reductions over 12 months, with a greater proportion of patients achieving >20% ALP declines. These findings support that targeted immunomodulation, particularly via JAK/STAT pathway inhibition, may have therapeutic potential in altering disease activity in PSC.
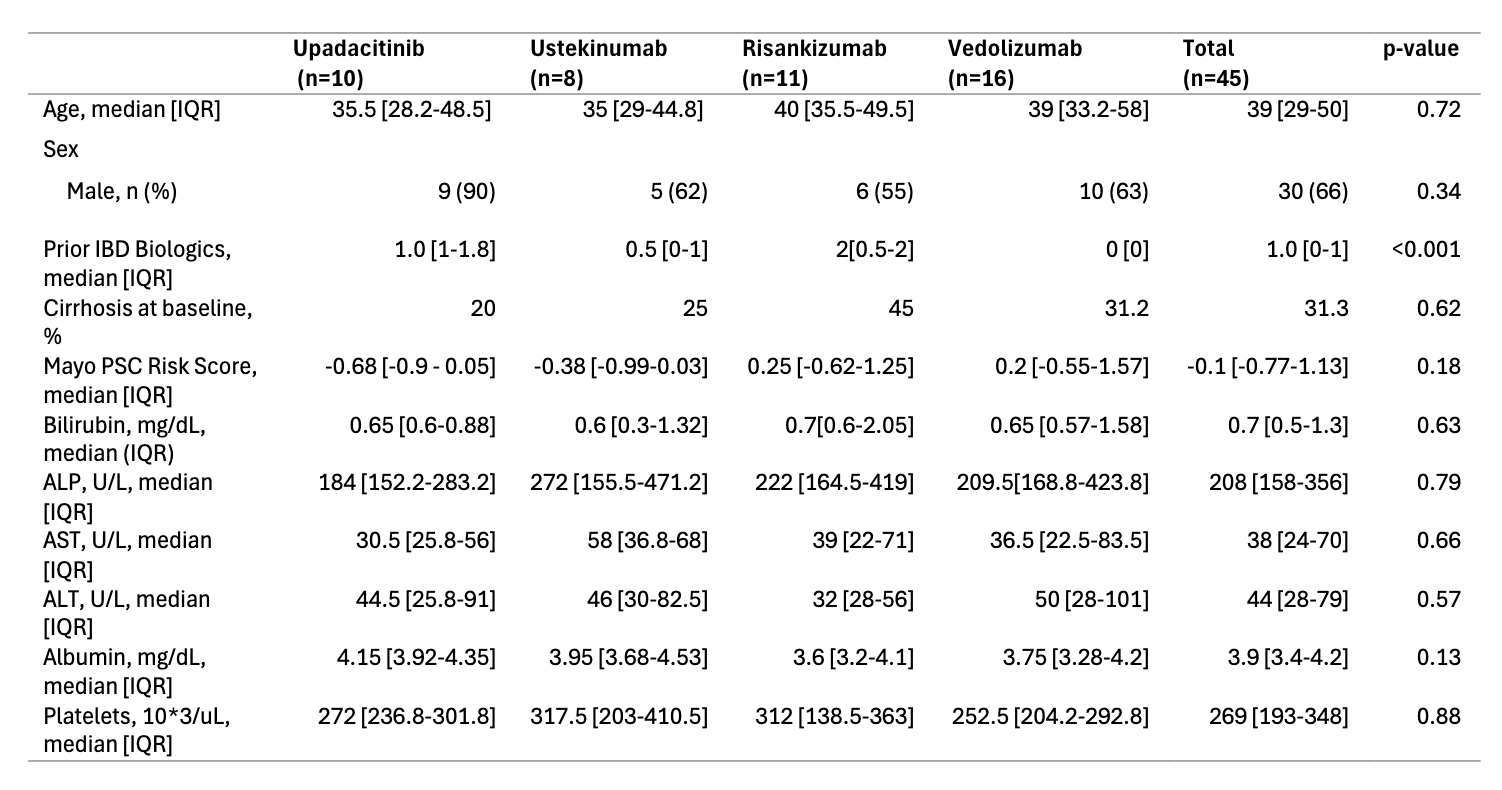
Figure: Table 1. Baseline Demographics, Clinical, and Laboratory Data
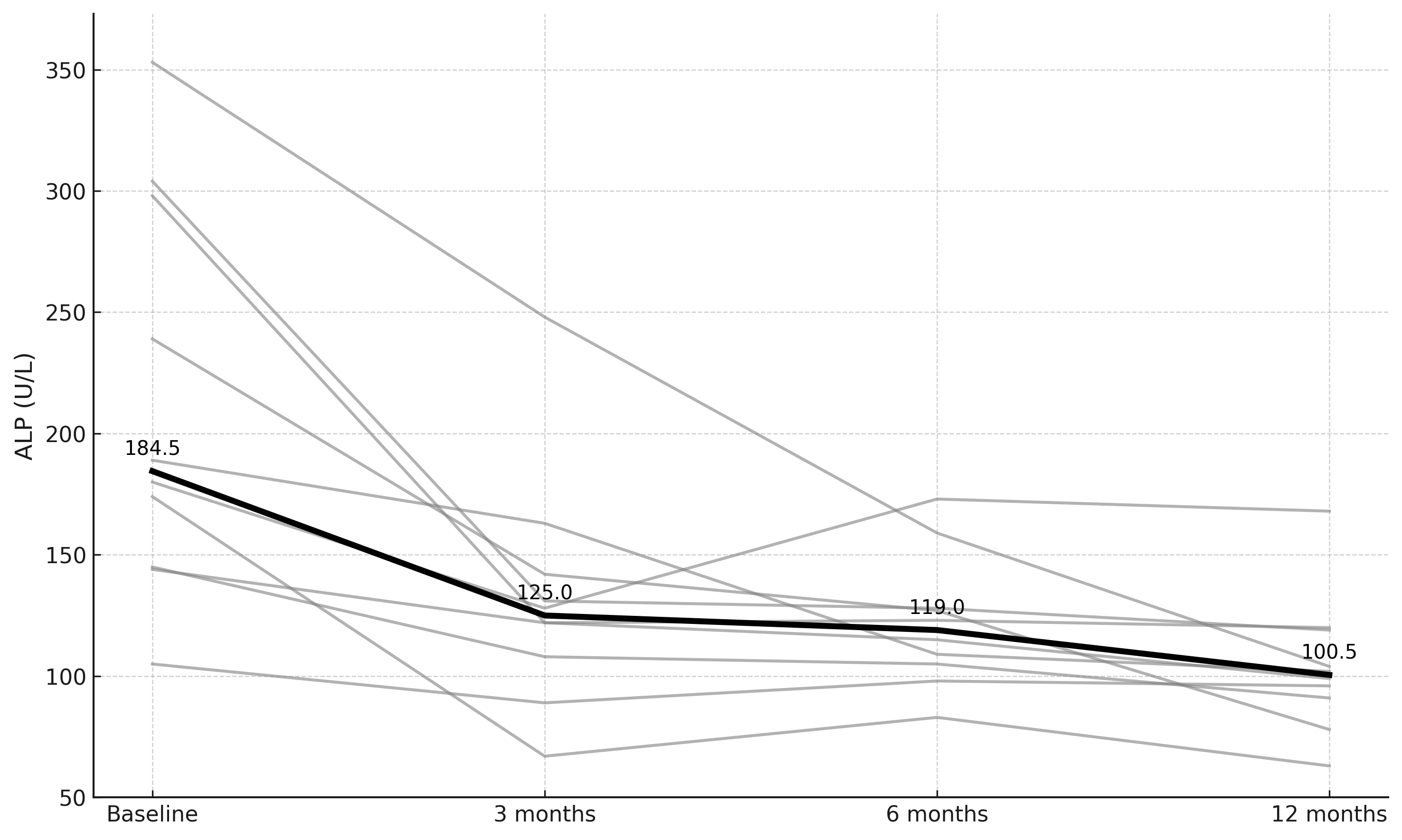
Figure: Figure 1. Longitudinal changes in alkaline phosphatase among 10 patients treated with upadacitinib over 12 months. Gray lines represent individual patients; the bold line indicates the group median at each time point.
Disclosures:
Benjamin Robinson indicated no relevant financial relationships.
Patrick Green indicated no relevant financial relationships.
Maithili Chitnavis: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Eli Lilly – Speakers Bureau.
Andrew DeLemos indicated no relevant financial relationships.
Benjamin L. Robinson, DO1, Patrick D. Green, MD2, Maithili V. Chitnavis, MD, FACG3, Andrew S. DeLemos, MD3. P5930 - Evaluating the Role of Advanced Biologic Therapies on Liver Biochemistry in Concurrent Primary Sclerosing Cholangitis and Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Benjamin L. Robinson, DO1, Patrick D. Green, MD2, Maithili V. Chitnavis, MD, FACG3, Andrew S. DeLemos, MD3
1Atrium Health Carolinas Medical Center, Charlotte, NC; 2Wake Forest Baptist Health, Winston-Salem, NC; 3Atrium Health, Charlotte, NC
Introduction: The overlap between primary sclerosing cholangitis (PSC) and inflammatory bowel disease (IBD) suggests a shared immunopathogenesis, raising the possibility that targeting key inflammatory pathways may modify the course of both diseases. With conventional biologics showing limited impact on PSC, this study explores the potential of novel IBD-directed therapies to influence disease trajectory in PSC-IBD.
Methods: We conducted a multi-center, retrospective cohort study of adult patients with PSC-IBD treated with upadacitinib, ustekinumab, risankizumab, or vedolizumab, using patient data between 01/2019 and 12/2024. Baseline demographic, clinical, and biochemical data were collected (Table 1). Liver biochemistries and available magnetic resonance cholangiopancreatography (MRCP) imaging were analyzed over 12 months.
Results: Data were collected from 45 patients with PSC-IBD: upadacitinib (n=10), ustekinumab (n=8), risankizumab (n=11), and vedolizumab (n=16). Across cohorts, most patients were white (75%) males (66%) with ulcerative colitis (70.6%) and a median PSC duration of 5 years (IQR 3–6.5). At baseline, 34.8% were receiving ursodeoxycholic acid, and 31.3% had cirrhosis, with a median Mayo PSC Risk Score of –0.1 (IQR –0.77 to 1.13). Large duct PSC was more common (76%) than small duct PSC, and 18% had autoimmune hepatitis (AIH) overlap. In the upadacitinib group, median ALP declined significantly from 184.5 U/L (IQR 152.2–283.2) at baseline to 125 (111.5–139.2) at 3 months, 119 (106–127.8) at 6 months, and 100.5 (92.2–115.2) at 12 months (Figure 1, p = 0.01). No other treatment groups showed significant ALP changes over time, and no significant differences in median AST, ALT, bilirubin, or platelets were observed across any groups from baseline to 12 months. The proportion of patients achieving a >20% reduction in ALP by 12 months was 80% with upadacitinib, 50% with ustekinumab, 63% with risankizumab, and 19% with vedolizumab. Among patients with follow-up MRCP at least 3 months after treatment initiation (n=23), upadacitinib was the only treatment group in which a patient showed radiographic improvement in biliary stricturing and ductal dilation compared to baseline.
Discussion: Upadacitinib was associated with significant ALP reductions over 12 months, with a greater proportion of patients achieving >20% ALP declines. These findings support that targeted immunomodulation, particularly via JAK/STAT pathway inhibition, may have therapeutic potential in altering disease activity in PSC.

Figure: Table 1. Baseline Demographics, Clinical, and Laboratory Data

Figure: Figure 1. Longitudinal changes in alkaline phosphatase among 10 patients treated with upadacitinib over 12 months. Gray lines represent individual patients; the bold line indicates the group median at each time point.
Disclosures:
Benjamin Robinson indicated no relevant financial relationships.
Patrick Green indicated no relevant financial relationships.
Maithili Chitnavis: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Eli Lilly – Speakers Bureau.
Andrew DeLemos indicated no relevant financial relationships.
Benjamin L. Robinson, DO1, Patrick D. Green, MD2, Maithili V. Chitnavis, MD, FACG3, Andrew S. DeLemos, MD3. P5930 - Evaluating the Role of Advanced Biologic Therapies on Liver Biochemistry in Concurrent Primary Sclerosing Cholangitis and Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

