Tuesday Poster Session
Category: Liver
P5928 - Longitudinal Trends in Liver Enzymes, Lipid Profiles, Thyroid Function, and BMI in MASLD Patients Treated With Resmetirom: A Multicenter Retrospective Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- FT
Fadi Totah, DO
Riverside Community Hospital
Riverside, CA
Presenting Author(s)
Fadi Totah, DO1, Regis Lee, DO2, Pejman Solaimani, MD3
1Riverside Community Hospital, Riverside, CA; 2HCA Healthcare Riverside Community Hospital, Riverside, CA; 3HCA Riverside Community Hospital, Riverside, CA
Introduction: Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common cause of chronic liver disease globally, affecting nearly 30% of adults. A subset of patients progress to metabolic dysfunction-associated steatohepatitis (MASH), increasing the risk of fibrosis, cirrhosis, and hepatocellular carcinoma. In 2024, Resmetirom became the first FDA-approved therapy for MASH with moderate to advanced fibrosis. While clinical trials have shown benefits in liver fat, fibrosis markers, and lipid profiles, real-world data on its metabolic and biochemical effects remain limited. This multicenter study evaluates trends in liver enzymes, lipid levels, thyroid function, and BMI in patients treated with Resmetirom in routine practice over a 12 month period.
Methods: Using the TriNetX research network encompassing 33 healthcare organizations, we identified 1,254 patients with MASLD or metabolic dysfunction-associated steatohepatitis (MASH) prescribed Resmetirom using ICD-10 codes. We analyzed trends in mean laboratory values (± SD) at baseline and at 1, 3, 6, and 12 months following treatment initiation.
Results: Mean lab values showed favorable trends over time. ALT decreased from 59.8 to 51.6 U/L and AST from 45.5 to 40.8 U/L, despite a transient AST rise at 1 month (48.8 U/L). GGT peaked at 107.1 U/L at 1 month before declining to 63.1 U/L at 12 months. Lipid values remained stable: total cholesterol (151 to 150 mg/dL), LDL (79.6 to 80.9 mg/dL at 6 months, then returning to baseline), HDL (44.6 to 44.8 mg/dL), and triglycerides (159 to 148 mg/dL), with brief early elevations. TSH increased from 2.02 to 2.4 μIU/mL (peak 2.5 at 6 months), while Free T3 and Free T4 showed minor, non-directional variation. BMI declined from 31.9 to 28.6 kg/m², reflecting meaningful weight loss during therapy (Figure 1).
Discussion: In this real-world multicenter study, Resmetirom therapy was associated with sustained reductions in ALT, AST, and BMI, while lipid and thyroid parameters remained stable. An early rise in GGT was noted at 1 month, followed by a consistent decline, suggesting a transient hepatic response that warrants further investigation.
These findings support the use of Resmetirom in clinical practice as a well-tolerated agent that improves liver biochemistry and promotes weight reduction without adversely affecting lipid or thyroid function. Future research should incorporate noninvasive modalities—such as transient elastography—to assess changes in hepatic steatosis and fibrosis.
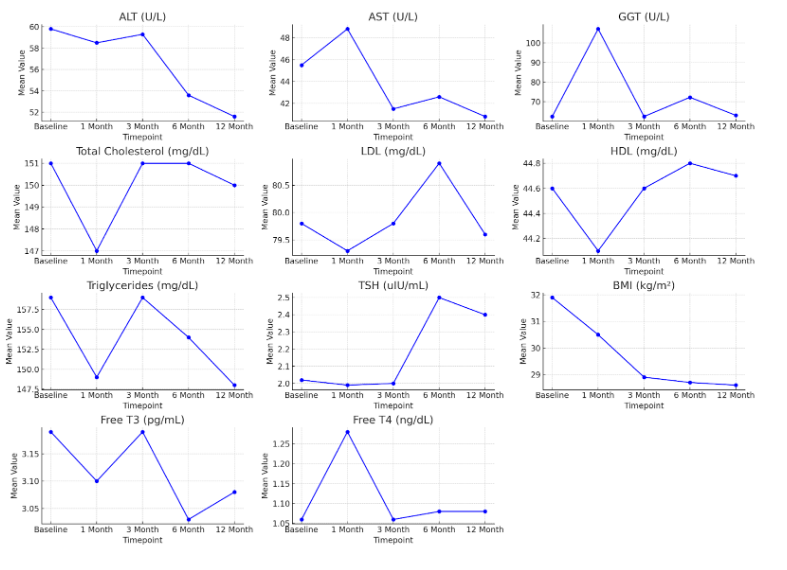
Figure: Figure 1. Mean Laboratory Marker Trends with Units Over 12 Months of Resmetirom Therapy
Disclosures:
Fadi Totah indicated no relevant financial relationships.
Regis Lee indicated no relevant financial relationships.
Pejman Solaimani indicated no relevant financial relationships.
Fadi Totah, DO1, Regis Lee, DO2, Pejman Solaimani, MD3. P5928 - Longitudinal Trends in Liver Enzymes, Lipid Profiles, Thyroid Function, and BMI in MASLD Patients Treated With Resmetirom: A Multicenter Retrospective Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Riverside Community Hospital, Riverside, CA; 2HCA Healthcare Riverside Community Hospital, Riverside, CA; 3HCA Riverside Community Hospital, Riverside, CA
Introduction: Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common cause of chronic liver disease globally, affecting nearly 30% of adults. A subset of patients progress to metabolic dysfunction-associated steatohepatitis (MASH), increasing the risk of fibrosis, cirrhosis, and hepatocellular carcinoma. In 2024, Resmetirom became the first FDA-approved therapy for MASH with moderate to advanced fibrosis. While clinical trials have shown benefits in liver fat, fibrosis markers, and lipid profiles, real-world data on its metabolic and biochemical effects remain limited. This multicenter study evaluates trends in liver enzymes, lipid levels, thyroid function, and BMI in patients treated with Resmetirom in routine practice over a 12 month period.
Methods: Using the TriNetX research network encompassing 33 healthcare organizations, we identified 1,254 patients with MASLD or metabolic dysfunction-associated steatohepatitis (MASH) prescribed Resmetirom using ICD-10 codes. We analyzed trends in mean laboratory values (± SD) at baseline and at 1, 3, 6, and 12 months following treatment initiation.
Results: Mean lab values showed favorable trends over time. ALT decreased from 59.8 to 51.6 U/L and AST from 45.5 to 40.8 U/L, despite a transient AST rise at 1 month (48.8 U/L). GGT peaked at 107.1 U/L at 1 month before declining to 63.1 U/L at 12 months. Lipid values remained stable: total cholesterol (151 to 150 mg/dL), LDL (79.6 to 80.9 mg/dL at 6 months, then returning to baseline), HDL (44.6 to 44.8 mg/dL), and triglycerides (159 to 148 mg/dL), with brief early elevations. TSH increased from 2.02 to 2.4 μIU/mL (peak 2.5 at 6 months), while Free T3 and Free T4 showed minor, non-directional variation. BMI declined from 31.9 to 28.6 kg/m², reflecting meaningful weight loss during therapy (Figure 1).
Discussion: In this real-world multicenter study, Resmetirom therapy was associated with sustained reductions in ALT, AST, and BMI, while lipid and thyroid parameters remained stable. An early rise in GGT was noted at 1 month, followed by a consistent decline, suggesting a transient hepatic response that warrants further investigation.
These findings support the use of Resmetirom in clinical practice as a well-tolerated agent that improves liver biochemistry and promotes weight reduction without adversely affecting lipid or thyroid function. Future research should incorporate noninvasive modalities—such as transient elastography—to assess changes in hepatic steatosis and fibrosis.

Figure: Figure 1. Mean Laboratory Marker Trends with Units Over 12 Months of Resmetirom Therapy
Disclosures:
Fadi Totah indicated no relevant financial relationships.
Regis Lee indicated no relevant financial relationships.
Pejman Solaimani indicated no relevant financial relationships.
Fadi Totah, DO1, Regis Lee, DO2, Pejman Solaimani, MD3. P5928 - Longitudinal Trends in Liver Enzymes, Lipid Profiles, Thyroid Function, and BMI in MASLD Patients Treated With Resmetirom: A Multicenter Retrospective Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
