Tuesday Poster Session
Category: Liver
P5899 - Distinct Lipid and Liver Enzyme Trajectories in Diabetic vs Non-Diabetic MASLD/MASH Patients Treated With Resmetirom: A Multicenter Propensity-Matched Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Regis Lee, DO
HCA Healthcare Riverside Community Hospital
Riverside, CA
Presenting Author(s)
Regis Lee, DO1, Fadi Totah, DO2, Pejman Solaimani, MD1
1HCA Healthcare Riverside Community Hospital, Riverside, CA; 2Riverside Community Hospital, Riverside, CA
Introduction: Metabolic dysfunction-associated steatotic liver disease (MASLD) is one of the most prevalent causes of chronic liver disease globally, driven in large part by its strong association with diabetes. With limited treatments available, resmetirom, a selective THR-β agonist, has shown potential in reducing liver fat and improving lipid profiles. Given that diabetes is a key driver of disease progression in MASLD, patients with diabetes may exhibit distinct metabolic and hepatic responses to resmetirom. This study evaluates resmetriom’s impact on liver enzymes and lipid profiles in MASLD and metabolic dysfunction-associated steatohepatitis (MASH) patients with and without diabetes.
Methods: Using the TriNetX database of 148 healthcare organizations, we identified 716 MASLD/MASH patients with diabetes (n=716) and without (n= 664) that were prescribed resmetirom. After propensity score matching for age, race, gender, BMI, and lipid-lowering agents, 461 patients remained in each group. We compared mean laboratory trends (± SD) at initiation of resmetirom and 1, 6, and 12 months after starting treatment between cohorts.
Results: Patients with diabetes treated with resmetirom had a statistically lower LDL and total cholesterol at all follow-up timepoints in this study (p< 0.05). However, HDL was not significantly different at each follow-up time point, while triglyceride levels were only statistically different only at the 1-month mark between the cohorts (p< 0.05)(Table 1). Liver enzymes (ALT and AST) showed different trajectories in this study with non-diabetic patients experiencing a transient increase at 1 month, whereas diabetics maintained stable levels, however this was not statistically significant (Figure 1). By 6 and 12 months, both groups improved, with a statistically significant difference in ALT and AST at 6 months (p< 0.05). Although GGT declined in both groups over 12 months, the greater reduction in diabetics was not statistically significant at each following time point.
Discussion: This study highlights distinct biochemical response patterns to resmetirom among MASLD/MASH patients based on diabetic status. Diabetic patients demonstrated more stable liver enzymes and sustained lipid reductions over 12 months. These findings underscore the potential for diabetes-specific treatment strategies in MASLD. Future research should explore whether underlying glycemic control, insulin resistance, or antidiabetic therapies modulate resmetirom's efficacy and can lead to improved outcomes.
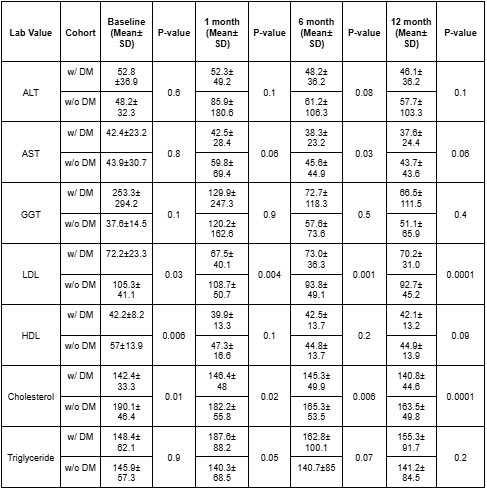
Figure: Table 1: Laboratory values in patients on Resmetirom with MASLD/MASH with and without diabetes.
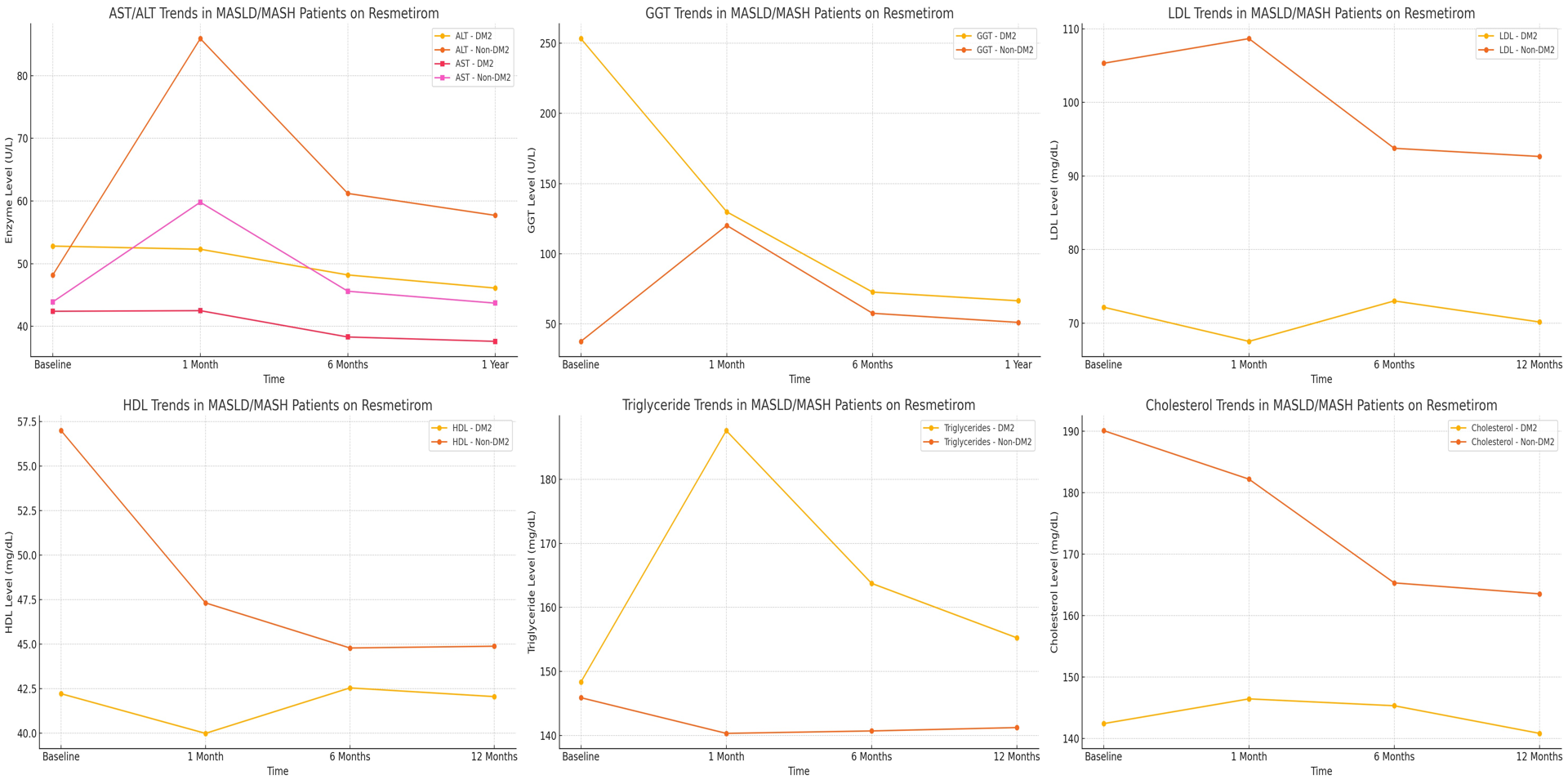
Figure: Figure 1: Trends of laboratory values in patients on Resmetirom with MASLD/MASH with and without diabetes.
Disclosures:
Regis Lee indicated no relevant financial relationships.
Fadi Totah indicated no relevant financial relationships.
Pejman Solaimani indicated no relevant financial relationships.
Regis Lee, DO1, Fadi Totah, DO2, Pejman Solaimani, MD1. P5899 - Distinct Lipid and Liver Enzyme Trajectories in Diabetic vs Non-Diabetic MASLD/MASH Patients Treated With Resmetirom: A Multicenter Propensity-Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1HCA Healthcare Riverside Community Hospital, Riverside, CA; 2Riverside Community Hospital, Riverside, CA
Introduction: Metabolic dysfunction-associated steatotic liver disease (MASLD) is one of the most prevalent causes of chronic liver disease globally, driven in large part by its strong association with diabetes. With limited treatments available, resmetirom, a selective THR-β agonist, has shown potential in reducing liver fat and improving lipid profiles. Given that diabetes is a key driver of disease progression in MASLD, patients with diabetes may exhibit distinct metabolic and hepatic responses to resmetirom. This study evaluates resmetriom’s impact on liver enzymes and lipid profiles in MASLD and metabolic dysfunction-associated steatohepatitis (MASH) patients with and without diabetes.
Methods: Using the TriNetX database of 148 healthcare organizations, we identified 716 MASLD/MASH patients with diabetes (n=716) and without (n= 664) that were prescribed resmetirom. After propensity score matching for age, race, gender, BMI, and lipid-lowering agents, 461 patients remained in each group. We compared mean laboratory trends (± SD) at initiation of resmetirom and 1, 6, and 12 months after starting treatment between cohorts.
Results: Patients with diabetes treated with resmetirom had a statistically lower LDL and total cholesterol at all follow-up timepoints in this study (p< 0.05). However, HDL was not significantly different at each follow-up time point, while triglyceride levels were only statistically different only at the 1-month mark between the cohorts (p< 0.05)(Table 1). Liver enzymes (ALT and AST) showed different trajectories in this study with non-diabetic patients experiencing a transient increase at 1 month, whereas diabetics maintained stable levels, however this was not statistically significant (Figure 1). By 6 and 12 months, both groups improved, with a statistically significant difference in ALT and AST at 6 months (p< 0.05). Although GGT declined in both groups over 12 months, the greater reduction in diabetics was not statistically significant at each following time point.
Discussion: This study highlights distinct biochemical response patterns to resmetirom among MASLD/MASH patients based on diabetic status. Diabetic patients demonstrated more stable liver enzymes and sustained lipid reductions over 12 months. These findings underscore the potential for diabetes-specific treatment strategies in MASLD. Future research should explore whether underlying glycemic control, insulin resistance, or antidiabetic therapies modulate resmetirom's efficacy and can lead to improved outcomes.

Figure: Table 1: Laboratory values in patients on Resmetirom with MASLD/MASH with and without diabetes.

Figure: Figure 1: Trends of laboratory values in patients on Resmetirom with MASLD/MASH with and without diabetes.
Disclosures:
Regis Lee indicated no relevant financial relationships.
Fadi Totah indicated no relevant financial relationships.
Pejman Solaimani indicated no relevant financial relationships.
Regis Lee, DO1, Fadi Totah, DO2, Pejman Solaimani, MD1. P5899 - Distinct Lipid and Liver Enzyme Trajectories in Diabetic vs Non-Diabetic MASLD/MASH Patients Treated With Resmetirom: A Multicenter Propensity-Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
