Tuesday Poster Session
Category: Liver
P5897 - Safety and Efficacy of Isolated Hepatic Perfusion With Melphalan + TNF-α for Advanced Liver Malignancies: A Systematic Review and Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Khadija Mohib, MD (she/her/hers)
Kirk Kerkorian School of Medicine at the University of Nevada Las Vegas
Las Vegas, NV
Presenting Author(s)
Khadija Mohib, MD1, Mirha Ali, MBBS2, Muhammad Ahsan, MBBS3, Abu-Bakr Ahmed, BA1, Manahil Ahmed, MBBS2, Barka Sajid, MBBS4, Syeda Qaima Abbas, MBBS2, Reenal Fairy, MBBS5, Zainab Wahaj, MBBS2
1Kirk Kerkorian School of Medicine at the University of Nevada Las Vegas, Las Vegas, NV; 2Department of Medicine, Jinnah Sindh Medical University, Karachi, Pakistan, Karachi, Sindh, Pakistan; 3Tabba Kidney Institute, Karachi, Sindh, Pakistan; 4Sindh Medical College, Karachi, Sindh, Pakistan; 5Department of Medicine, Jinnah Sindh Medical University, Karachi, Pakistan, Karachi, Sindh, Pakistan
Introduction: Surgical intervention is the primary treatment for liver malignancy, but two-thirds of patients are ineligible for resection. They are treated with Isolated Hepatic Perfusion (IHP), where high-dose chemotherapy, commonly Melphalan with TNF-α, is administered directly to the liver, limiting systemic toxicity. Techniques like Percutaneous Hepatic Perfusion (PHP) and Trans arterial Chemoembolization (TACE) are suboptimal for advanced tumor burdens. IHP is used at specialized centers, but minimal sample size and procedural complexity limit data. This is the first ever meta-analysis to assess the safety of IHP on tumor response, survival, and toxicity.
Methods: A literature search from inception till April 2025 was performed using PubMed, Cochrane Library, Google Scholar, and ClinicalTrials.gov. Non-randomized single-arm trials were screened. Eligible studies involved adults over 18 undergoing IHP with Melphalan+TNF-α for primary liver malignancies or secondary unresectable liver metastases. Meta-analysis and subgroup analysis were done using Comprehensive Meta-Analysis software with 95% confidence intervals (CI). Risk of bias was assessed with ROBINS-I and certainty of evidence was evaluated using GRADEpro. Sensitivity analyses were done for outcomes with heterogeneity exceeding I² > 50.
Results: Eight non-overlapping studies (155 patients) were included. For primary outcomes, partial response was seen in 56.7% (95% CI: 42.5%-69.9%, p< 0.001; I²=54.8%), and complete response in 9.0% (95% CI: 2.1%-31.7%, p=0.044; I²=67.9%). Overall response rate was 66.2% (95% CI: 57.1%-74.2%, p=0.341; I²=11.6%). For secondary outcomes, grade 3–4 hematologic toxicity was less common 22.0% (95% CI: 12.0%-39.4%, p=0.448; I²=0%), while hepatic toxicity affected 69.1% (95% CI: 58.7%-77.8%, p=0.568; I²=0%). Mean hospital stay was 18.5 days (95% CI: 11.3–25.7, p< 0.0001; I²=95%). Subgroup for tumor type (neuroendocrine vs non-neuroendocrine) and age group (≥55 vs < 55) did not alter response or hepatic toxicity.
Discussion: IHP with Melphalan ± TNF-α shows moderate tumor response and promising survival in advanced liver malignancies but carries high hepatic toxicity and procedural burden. These findings offer direction, yet underline the urgent need for larger, controlled trials, to treat unresectable tumor metastasis.
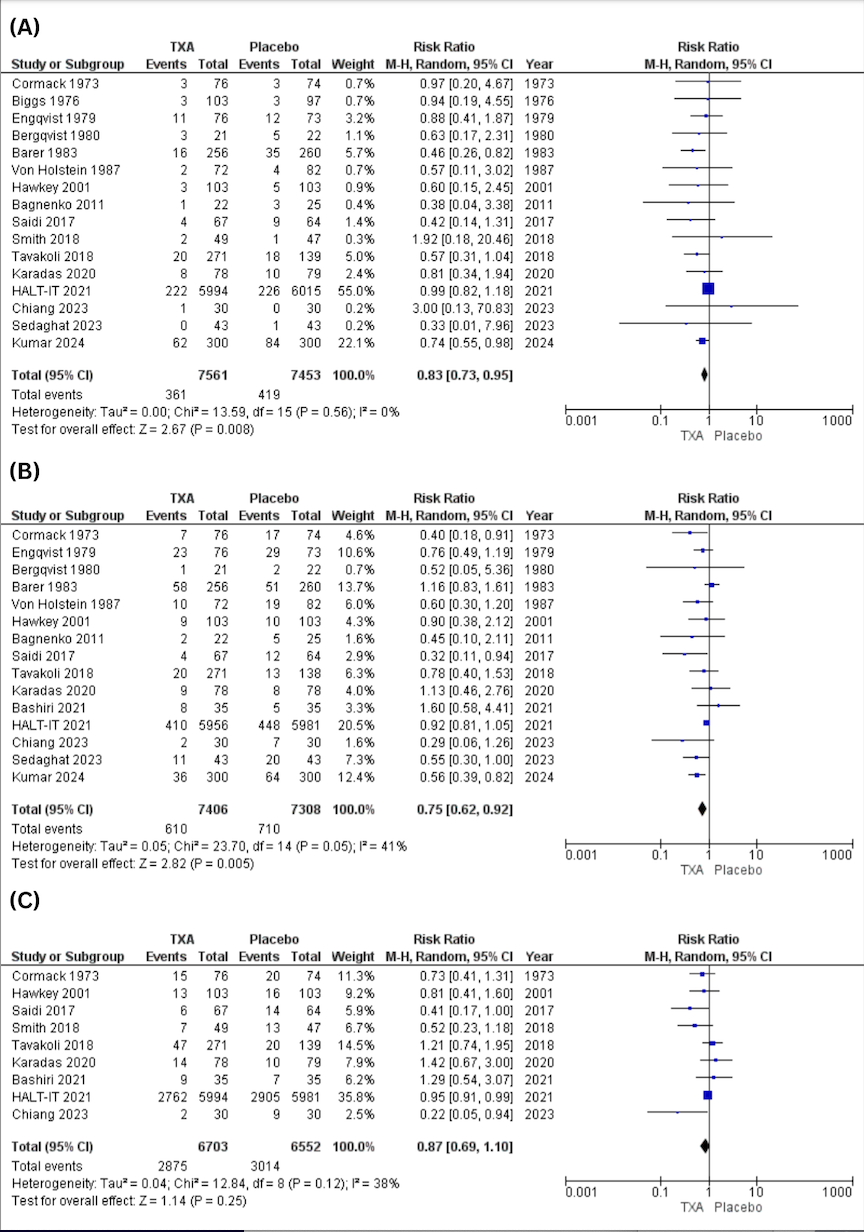
Figure: Figure A1
Primary Outcome: Partial Response
Figure A2:
Primary Outcome: Complete Response
Figure A3:
Primary Outcome: Overall Response Rate
Figure B1:
Secondary Outcome: Grade 3-4 Hepatic Toxicity
Figure B2:
Secondary Outcome: Grade 3-4 Hematological Toxicity
Figure C: Tumor Type Subgroup: Overall Response Rate
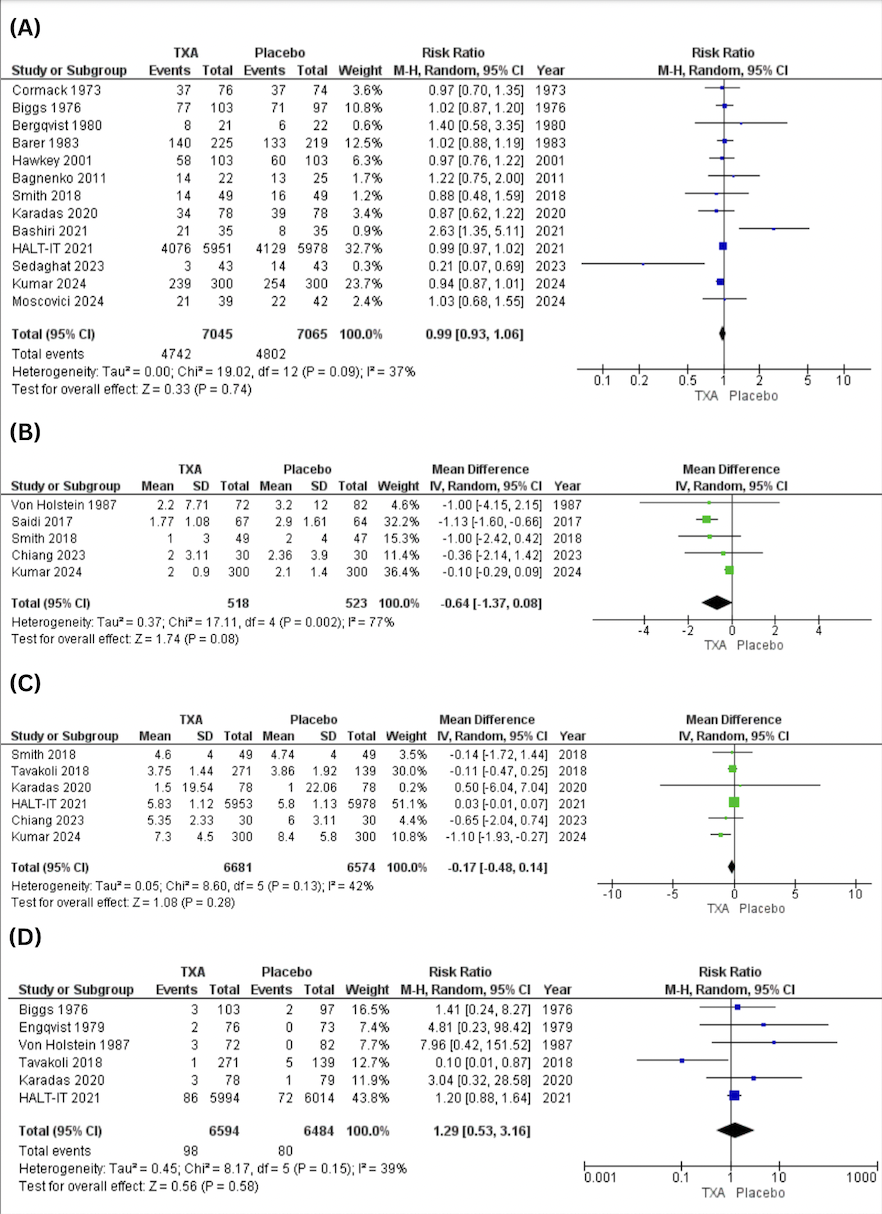
Figure: Figure D: Grade 3-4 Hepatic Toxicity by Age Subgroup ( <55 vs 55 and above years)
Figure E: Risk of Bias Assessment using ROBINS-1
Figure F: GRADE Assessment of each Outcome
Disclosures:
Khadija Mohib indicated no relevant financial relationships.
Mirha Ali indicated no relevant financial relationships.
Muhammad Ahsan indicated no relevant financial relationships.
Abu-Bakr Ahmed indicated no relevant financial relationships.
Manahil Ahmed indicated no relevant financial relationships.
Barka Sajid indicated no relevant financial relationships.
Syeda Qaima Abbas indicated no relevant financial relationships.
Reenal Fairy indicated no relevant financial relationships.
Zainab Wahaj indicated no relevant financial relationships.
Khadija Mohib, MD1, Mirha Ali, MBBS2, Muhammad Ahsan, MBBS3, Abu-Bakr Ahmed, BA1, Manahil Ahmed, MBBS2, Barka Sajid, MBBS4, Syeda Qaima Abbas, MBBS2, Reenal Fairy, MBBS5, Zainab Wahaj, MBBS2. P5897 - Safety and Efficacy of Isolated Hepatic Perfusion With Melphalan + TNF-α for Advanced Liver Malignancies: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Kirk Kerkorian School of Medicine at the University of Nevada Las Vegas, Las Vegas, NV; 2Department of Medicine, Jinnah Sindh Medical University, Karachi, Pakistan, Karachi, Sindh, Pakistan; 3Tabba Kidney Institute, Karachi, Sindh, Pakistan; 4Sindh Medical College, Karachi, Sindh, Pakistan; 5Department of Medicine, Jinnah Sindh Medical University, Karachi, Pakistan, Karachi, Sindh, Pakistan
Introduction: Surgical intervention is the primary treatment for liver malignancy, but two-thirds of patients are ineligible for resection. They are treated with Isolated Hepatic Perfusion (IHP), where high-dose chemotherapy, commonly Melphalan with TNF-α, is administered directly to the liver, limiting systemic toxicity. Techniques like Percutaneous Hepatic Perfusion (PHP) and Trans arterial Chemoembolization (TACE) are suboptimal for advanced tumor burdens. IHP is used at specialized centers, but minimal sample size and procedural complexity limit data. This is the first ever meta-analysis to assess the safety of IHP on tumor response, survival, and toxicity.
Methods: A literature search from inception till April 2025 was performed using PubMed, Cochrane Library, Google Scholar, and ClinicalTrials.gov. Non-randomized single-arm trials were screened. Eligible studies involved adults over 18 undergoing IHP with Melphalan+TNF-α for primary liver malignancies or secondary unresectable liver metastases. Meta-analysis and subgroup analysis were done using Comprehensive Meta-Analysis software with 95% confidence intervals (CI). Risk of bias was assessed with ROBINS-I and certainty of evidence was evaluated using GRADEpro. Sensitivity analyses were done for outcomes with heterogeneity exceeding I² > 50.
Results: Eight non-overlapping studies (155 patients) were included. For primary outcomes, partial response was seen in 56.7% (95% CI: 42.5%-69.9%, p< 0.001; I²=54.8%), and complete response in 9.0% (95% CI: 2.1%-31.7%, p=0.044; I²=67.9%). Overall response rate was 66.2% (95% CI: 57.1%-74.2%, p=0.341; I²=11.6%). For secondary outcomes, grade 3–4 hematologic toxicity was less common 22.0% (95% CI: 12.0%-39.4%, p=0.448; I²=0%), while hepatic toxicity affected 69.1% (95% CI: 58.7%-77.8%, p=0.568; I²=0%). Mean hospital stay was 18.5 days (95% CI: 11.3–25.7, p< 0.0001; I²=95%). Subgroup for tumor type (neuroendocrine vs non-neuroendocrine) and age group (≥55 vs < 55) did not alter response or hepatic toxicity.
Discussion: IHP with Melphalan ± TNF-α shows moderate tumor response and promising survival in advanced liver malignancies but carries high hepatic toxicity and procedural burden. These findings offer direction, yet underline the urgent need for larger, controlled trials, to treat unresectable tumor metastasis.

Figure: Figure A1
Primary Outcome: Partial Response
Figure A2:
Primary Outcome: Complete Response
Figure A3:
Primary Outcome: Overall Response Rate
Figure B1:
Secondary Outcome: Grade 3-4 Hepatic Toxicity
Figure B2:
Secondary Outcome: Grade 3-4 Hematological Toxicity
Figure C: Tumor Type Subgroup: Overall Response Rate

Figure: Figure D: Grade 3-4 Hepatic Toxicity by Age Subgroup ( <55 vs 55 and above years)
Figure E: Risk of Bias Assessment using ROBINS-1
Figure F: GRADE Assessment of each Outcome
Disclosures:
Khadija Mohib indicated no relevant financial relationships.
Mirha Ali indicated no relevant financial relationships.
Muhammad Ahsan indicated no relevant financial relationships.
Abu-Bakr Ahmed indicated no relevant financial relationships.
Manahil Ahmed indicated no relevant financial relationships.
Barka Sajid indicated no relevant financial relationships.
Syeda Qaima Abbas indicated no relevant financial relationships.
Reenal Fairy indicated no relevant financial relationships.
Zainab Wahaj indicated no relevant financial relationships.
Khadija Mohib, MD1, Mirha Ali, MBBS2, Muhammad Ahsan, MBBS3, Abu-Bakr Ahmed, BA1, Manahil Ahmed, MBBS2, Barka Sajid, MBBS4, Syeda Qaima Abbas, MBBS2, Reenal Fairy, MBBS5, Zainab Wahaj, MBBS2. P5897 - Safety and Efficacy of Isolated Hepatic Perfusion With Melphalan + TNF-α for Advanced Liver Malignancies: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
