Tuesday Poster Session
Category: Liver
P5859 - Terlipressin Is Associated With Decreased Renal Replacement Therapy Compared With Midodrine/Octreotide Among Patients With Hepatorenal Syndrome
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Tanvi Goyal, MD, MPH
Penn Medicine
Philadelphia, PA
Presenting Author(s)
Tanvi Goyal, MD, MPH1, Tushar Khanna, MD2, David Kaplan, MD2, Jonathan Nahas, MD2
1Penn Medicine, Philadelphia, PA; 2University of Pennsylvania/Penn Medicine, Philadelphia, PA
Introduction: Acute kidney-injury/Hepatorenal syndrome (HRS-AKI), a further decompensation of advanced chronic liver disease with portal hypertension, frequently requires initiation of renal replacement therapy (RRT) and is associated with significant mortality. Terlipressin (T), a synthetic vasopressin analogue, became the first FDA-approved treatment for HRS-AKI in 2022. The aim of this study was to evaluate differences in outcomes between patients with HRS-AKI on T versus the prior off-label standard of care midodrine/octreotide (MO) in a large real-world cohort.
Methods: We performed a comparative cohort study utilizing the US Collaborative TriNetX data base comprising retrospective data from 71 health care organizations. We included patients with a diagnosis of HRS-AKI treated with either T or MO with an inpatient, emergency or ICU visit within one day of diagnosis. Key covariates included: gender, race, heart failure, SIRS, ascites, hepatic encephalopathy, variceal hemorrhage, and labs including creatinine, INR, albumin, liver tests, and sodium. Primary endpoints included risk of requiring dialysis defined by the presence of codes for dialysis, liver transplantation and death. Data was analyzed using chi-square, t-test and Kaplan-Meier analysis.
Results: 10,832 MO and 151 T patients with a diagnosis of HRS-AKI within 1 day of treatment were identified. The mean ages for the MO and T groups were 55.4 and 55.6 years, respectively. 58.1% of the MO group and 63.6% of the T group were male, and 73.1% of the MO group and 74.8% of the T group were white. The average initial creatinine for the MO group was 3.21±1.79 mg/dL vs. 3.16 ±1.23 mg/dL for the T group, p=0.84. The risk of requiring renal replacement therapy (RRT) within 30 days was significantly lower in T group, 17.9% vs. 25.3%, p = 0.037. At 30 days, there were no differences in rates of liver transplantation or mortality (9.3% v 9.4%, 32.5 vs. 33.7% for T and MO, respectively). When 1:1 propensity matched, 151 MO and T patients were included. In this analysis, there was a trend towards less risk of RRT in the T group 17.9 % vs. 25.8% (p=0.09) at 1 month.
Discussion: Terlipressin appears to be associated with decreased rates of RRT when compared with midodrine/octreotide but similar rates of overall survival and liver transplantation in a real world US cohort. Ongoing work will assess the differential impact of terlipressin effect on hospital utilization, length of stay, and overall cost.
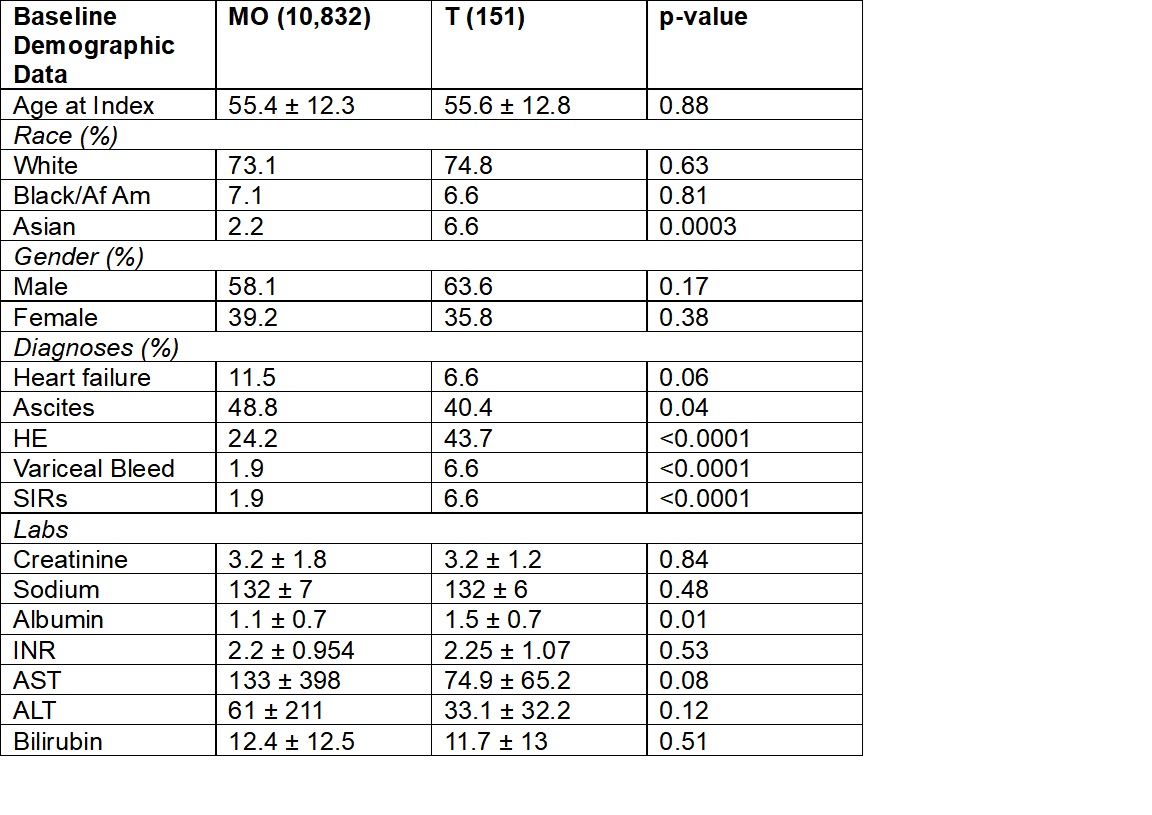
Figure: Table 1. Key Baseline Characteristics with Covariates Prior to 1:1 Propensity Matching
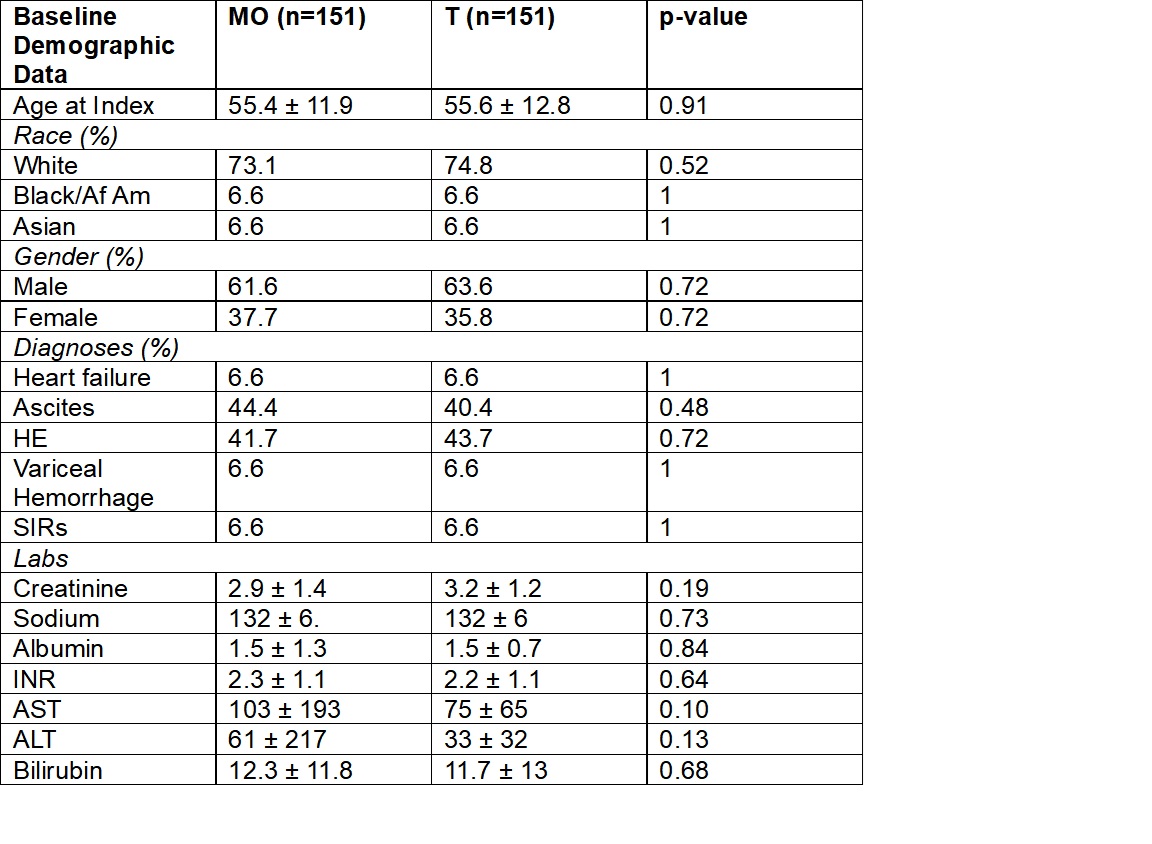
Figure: Table 2. Key Baseline Characteristics with Covariates After 1:1 Propensity Matching
Disclosures:
Tanvi Goyal indicated no relevant financial relationships.
Tushar Khanna indicated no relevant financial relationships.
David Kaplan indicated no relevant financial relationships.
Jonathan Nahas indicated no relevant financial relationships.
Tanvi Goyal, MD, MPH1, Tushar Khanna, MD2, David Kaplan, MD2, Jonathan Nahas, MD2. P5859 - Terlipressin Is Associated With Decreased Renal Replacement Therapy Compared With Midodrine/Octreotide Among Patients With Hepatorenal Syndrome, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Penn Medicine, Philadelphia, PA; 2University of Pennsylvania/Penn Medicine, Philadelphia, PA
Introduction: Acute kidney-injury/Hepatorenal syndrome (HRS-AKI), a further decompensation of advanced chronic liver disease with portal hypertension, frequently requires initiation of renal replacement therapy (RRT) and is associated with significant mortality. Terlipressin (T), a synthetic vasopressin analogue, became the first FDA-approved treatment for HRS-AKI in 2022. The aim of this study was to evaluate differences in outcomes between patients with HRS-AKI on T versus the prior off-label standard of care midodrine/octreotide (MO) in a large real-world cohort.
Methods: We performed a comparative cohort study utilizing the US Collaborative TriNetX data base comprising retrospective data from 71 health care organizations. We included patients with a diagnosis of HRS-AKI treated with either T or MO with an inpatient, emergency or ICU visit within one day of diagnosis. Key covariates included: gender, race, heart failure, SIRS, ascites, hepatic encephalopathy, variceal hemorrhage, and labs including creatinine, INR, albumin, liver tests, and sodium. Primary endpoints included risk of requiring dialysis defined by the presence of codes for dialysis, liver transplantation and death. Data was analyzed using chi-square, t-test and Kaplan-Meier analysis.
Results: 10,832 MO and 151 T patients with a diagnosis of HRS-AKI within 1 day of treatment were identified. The mean ages for the MO and T groups were 55.4 and 55.6 years, respectively. 58.1% of the MO group and 63.6% of the T group were male, and 73.1% of the MO group and 74.8% of the T group were white. The average initial creatinine for the MO group was 3.21±1.79 mg/dL vs. 3.16 ±1.23 mg/dL for the T group, p=0.84. The risk of requiring renal replacement therapy (RRT) within 30 days was significantly lower in T group, 17.9% vs. 25.3%, p = 0.037. At 30 days, there were no differences in rates of liver transplantation or mortality (9.3% v 9.4%, 32.5 vs. 33.7% for T and MO, respectively). When 1:1 propensity matched, 151 MO and T patients were included. In this analysis, there was a trend towards less risk of RRT in the T group 17.9 % vs. 25.8% (p=0.09) at 1 month.
Discussion: Terlipressin appears to be associated with decreased rates of RRT when compared with midodrine/octreotide but similar rates of overall survival and liver transplantation in a real world US cohort. Ongoing work will assess the differential impact of terlipressin effect on hospital utilization, length of stay, and overall cost.

Figure: Table 1. Key Baseline Characteristics with Covariates Prior to 1:1 Propensity Matching

Figure: Table 2. Key Baseline Characteristics with Covariates After 1:1 Propensity Matching
Disclosures:
Tanvi Goyal indicated no relevant financial relationships.
Tushar Khanna indicated no relevant financial relationships.
David Kaplan indicated no relevant financial relationships.
Jonathan Nahas indicated no relevant financial relationships.
Tanvi Goyal, MD, MPH1, Tushar Khanna, MD2, David Kaplan, MD2, Jonathan Nahas, MD2. P5859 - Terlipressin Is Associated With Decreased Renal Replacement Therapy Compared With Midodrine/Octreotide Among Patients With Hepatorenal Syndrome, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
