Tuesday Poster Session
Category: Liver
P5855 - Alcohol Use Reduction With GLP-1 Agonist Use
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Nishani Wickramarachchi, DO
HCA Healthcare Riverside Community Hospital
Riverside, CA
Presenting Author(s)
Nishani Wickramarachchi, DO1, Anthony Sobero, DO2, Alex Prevallet, DO1, Crystal Dorgalli, DO, MS3, Omar Shahbaz, MD, MS2
1HCA Healthcare Riverside Community Hospital, Riverside, CA; 2Riverside Community Hospital, Riverside, CA; 3HCA Riverside Community Hospital, Riverside, CA
Introduction: The therapeutic potential of glucagon-like peptide-1 (GLP-1) analogues in managing alcohol use disorders and related liver conditions has garnered increasing attention in recent years. Evidence suggests that GLP-1 plays a significant role in modulating alcohol-seeking behavior and consumption. This study aims to assess the effects of GLP-1 treatment in patients with alcohol use disorders and associated liver disease.
Methods: This retrospective cohort study utilized data from the TriNetX US Collaborative Network. Inclusion criteria comprised alcohol abuse or dependence, and alcoholic hepatitis or fatty liver. The treatment cohort received GLP-1 agonists within 5 years of diagnosis. Exclusion criteria included cirrhosis, autoimmune hepatitis, Wilson’s disease, hemochromatosis, and viral hepatitis. Propensity score matching balanced the cohorts by demographic and clinical characteristics, resulting in 4,533 patients per group. Outcomes within 5 years were analyzed using relative risk, risk difference, odds ratio, number of instances, and Kaplan-Meier survival analysis. Primary outcomes included hospitalization, alcoholic hepatitis, cirrhosis, mortality, and acute decompensated liver failure.
Results: The mean follow-up time was 681.4 days in the GLP-1 cohort and 517.2 days in the non-GLP-1 cohort. The GLP-1 group showed a significantly lower hospitalization risk (35.5% vs. 51.6%, risk difference = -0.161, p< 0.001). Kaplan-Meier analysis showed longer median survival in the GLP-1 cohort (1,144 days vs. 311 days, p< 0.001). The hazard ratio for survival was 0.479 (95% CI: 0.449–0.511, p< 0.001), indicating improved survival. The GLP-1 cohort also had less liver-related outcomes, including reduced rates of acute decompensated liver failure and alcoholic hepatitis, with no significant difference in cirrhosis rates.
Discussion: Animal studies suggest GLP-1 analogues may reduce alcohol use, though human data remains inconsistent. The findings in this study reveal that GLP-1 treatment in patients with alcohol use, alcoholic hepatitis, and fatty liver is associated with less hepatic disease progression, which could be seen as a proxy for reduced alcohol use and possible reduction of hepatic steatosis. GLP-1 therapy correlated with less alcoholic hepatitis. As patients continuing alcohol use face greater risks of cirrhosis and liver failure, GLP-1’s potential benefits are notable. More research is needed to clarify the mechanisms and long-term effects of GLP-1 agonists on liver disease progression.
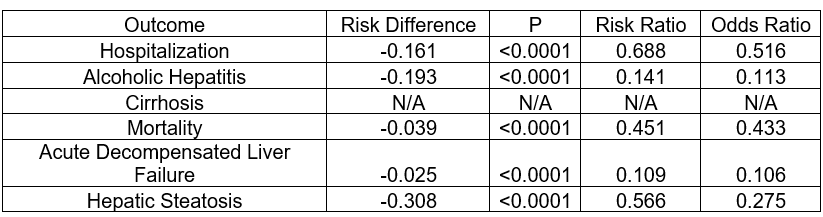
Figure: Table 1: Risk analysis of GLP-1 and non-GLP-1 groups in each outcome
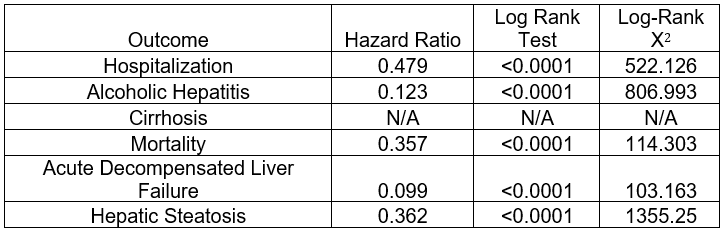
Figure: Table 2: Kaplan-Meier survival analysis of GLP-1 and non-GLP-1 groups in each outcome
Disclosures:
Nishani Wickramarachchi indicated no relevant financial relationships.
Anthony Sobero indicated no relevant financial relationships.
Alex Prevallet indicated no relevant financial relationships.
Crystal Dorgalli indicated no relevant financial relationships.
Omar Shahbaz indicated no relevant financial relationships.
Nishani Wickramarachchi, DO1, Anthony Sobero, DO2, Alex Prevallet, DO1, Crystal Dorgalli, DO, MS3, Omar Shahbaz, MD, MS2. P5855 - Alcohol Use Reduction With GLP-1 Agonist Use, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1HCA Healthcare Riverside Community Hospital, Riverside, CA; 2Riverside Community Hospital, Riverside, CA; 3HCA Riverside Community Hospital, Riverside, CA
Introduction: The therapeutic potential of glucagon-like peptide-1 (GLP-1) analogues in managing alcohol use disorders and related liver conditions has garnered increasing attention in recent years. Evidence suggests that GLP-1 plays a significant role in modulating alcohol-seeking behavior and consumption. This study aims to assess the effects of GLP-1 treatment in patients with alcohol use disorders and associated liver disease.
Methods: This retrospective cohort study utilized data from the TriNetX US Collaborative Network. Inclusion criteria comprised alcohol abuse or dependence, and alcoholic hepatitis or fatty liver. The treatment cohort received GLP-1 agonists within 5 years of diagnosis. Exclusion criteria included cirrhosis, autoimmune hepatitis, Wilson’s disease, hemochromatosis, and viral hepatitis. Propensity score matching balanced the cohorts by demographic and clinical characteristics, resulting in 4,533 patients per group. Outcomes within 5 years were analyzed using relative risk, risk difference, odds ratio, number of instances, and Kaplan-Meier survival analysis. Primary outcomes included hospitalization, alcoholic hepatitis, cirrhosis, mortality, and acute decompensated liver failure.
Results: The mean follow-up time was 681.4 days in the GLP-1 cohort and 517.2 days in the non-GLP-1 cohort. The GLP-1 group showed a significantly lower hospitalization risk (35.5% vs. 51.6%, risk difference = -0.161, p< 0.001). Kaplan-Meier analysis showed longer median survival in the GLP-1 cohort (1,144 days vs. 311 days, p< 0.001). The hazard ratio for survival was 0.479 (95% CI: 0.449–0.511, p< 0.001), indicating improved survival. The GLP-1 cohort also had less liver-related outcomes, including reduced rates of acute decompensated liver failure and alcoholic hepatitis, with no significant difference in cirrhosis rates.
Discussion: Animal studies suggest GLP-1 analogues may reduce alcohol use, though human data remains inconsistent. The findings in this study reveal that GLP-1 treatment in patients with alcohol use, alcoholic hepatitis, and fatty liver is associated with less hepatic disease progression, which could be seen as a proxy for reduced alcohol use and possible reduction of hepatic steatosis. GLP-1 therapy correlated with less alcoholic hepatitis. As patients continuing alcohol use face greater risks of cirrhosis and liver failure, GLP-1’s potential benefits are notable. More research is needed to clarify the mechanisms and long-term effects of GLP-1 agonists on liver disease progression.

Figure: Table 1: Risk analysis of GLP-1 and non-GLP-1 groups in each outcome

Figure: Table 2: Kaplan-Meier survival analysis of GLP-1 and non-GLP-1 groups in each outcome
Disclosures:
Nishani Wickramarachchi indicated no relevant financial relationships.
Anthony Sobero indicated no relevant financial relationships.
Alex Prevallet indicated no relevant financial relationships.
Crystal Dorgalli indicated no relevant financial relationships.
Omar Shahbaz indicated no relevant financial relationships.
Nishani Wickramarachchi, DO1, Anthony Sobero, DO2, Alex Prevallet, DO1, Crystal Dorgalli, DO, MS3, Omar Shahbaz, MD, MS2. P5855 - Alcohol Use Reduction With GLP-1 Agonist Use, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
