Tuesday Poster Session
Category: Liver
P5830 - The Use of Non-Invasive Tests to Characterise the Total Baseline Population in the Phase 3 Essence Trial
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- LC
Laurent Castera, MD, PhD
Université Paris Cité, UMR1149 (CRI), INSERM
Paris, Ile-de-France, France
Presenting Author(s)
Laurent Castera, MD, PhD1, Kristiane Engebretsen, MD, PhD2, George B.. Goh, MBBS, MRCP (UK), M Med (Int Med), FAMS (Singapore)3, Elisabetta Bugianesi, MD, PhD4, Anna M.G.. Calí, MD, MSc2, Burak Demirel, MD2, Tomáš Koller, MD, PhD5, Niels Krarup, MSc2, Michelle T.. Long, MD, MSc2, Philip Newsome, MD, PhD6, Cláudia Oliveira, MD, PhD7, Vlad Ratziu, MD, PhD8, Mary E.. Rinella, MD9, Michael Roden, MD, PhD10, Giada Sebastiani, MD11, Arun J.. Sanyal, MD12
1Université Paris Cité, UMR1149 (CRI), INSERM, Paris, Ile-de-France, France; 2Novo Nordisk A/S, Copenhagen, Hovedstaden, Denmark; 3Department of Gastroenterology and Hepatology, Singapore General Hospital, Singapore, Singapore; 4Department of Medical Sciences, University of Turin, Turin, Piemonte, Italy; 5Subdivision of Gastroenterology and Hepatology, 5th Department of Internal Medicine, Comenius University Faculty of Medicine, University Hospital Bratislava, Bratislava, Bratislava, Slovakia; 6Roger Williams Institute of Liver Studies, Faculty of Life Sciences and Medicine, King’s College London and King’s College Hospital, London, England, United Kingdom; 7Departamento de Gastroenterologia, Hospital das Clínicas (LIM07) da Faculdade de Medicina da Universidade de São Paulo, Sao Paulo, Sao Paulo, Brazil; 8Sorbonne Université, Institute for Cardiometabolism and Nutrition, Hospital Pitié Salpêtrière, INSERM UMRS 1138 CRC, Paris, Ile-de-France, France; 9Division of Gastroenterology, Hepatology and Nutrition, University of Chicago, Chicago, IL; 10Department of Endocrinology and Diabetology, Medical Faculty and University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Dusseldorf, Nordrhein-Westfalen, Germany; 11Division of Gastroenterology and Hepatology, McGill University Health Centre, Montreal, PQ, Canada; 12Virginia Commonwealth University; Central Virginia Veterans Healthcare System, Richmond, VA
Introduction: ESSENCE is an ongoing phase 3 trial (NCT04822181) in participants with biopsy-defined, non-cirrhotic metabolic dysfunction-associated steatohepatitis (MASH).In part 1, the primary histological endpoints were met in the first 800 randomised participants after 72 weeks’ treatment with semaglutide 2.4 mg. The aim of this post hoc analysis was to characterise the total randomised, baseline population using selected non-invasive tests (NITs).
Methods: Randomised participants (N=1197) were characterised by NITs at baseline: vibration-controlled transient elastography liver stiffness measurement (VCTE LSM; FibroScan), enhanced liver fibrosis (ELF) score, FibroScan-aspartate aminotransferase (FAST) score and Fibrosis-4 index (FIB-4) score. Eligible participants had histologically defined MASH and liver fibrosis stage 2 or 3. Clinical NIT categories for moderate-to-advanced fibrosis were used to group participants, and descriptive statistics determined numbers of participants in each NIT category and demographics.
Results: Distribution of results is shown in Table 1. At baseline, 95.5% fulfilled NIT scores in ≥1 of the guideline-recommended ranges for FIB-4 (≥1.3–≤2.67), ELF (≥9.0–< 11.3) or VCTE LSM (≥8.0–< 20.0 kPa) (Table 2). Most had a FAST score ≥0.35 (82.4%) and 34.7% had a FIB-4 score < 1.3 (Table 1). As expected, participants with FIB-4 score < 1.3 were younger (mean age: 48.1 years) than those with FIB-4 score ≥1.3–≤2.67 (59.1 years) and >2.67 (63.7 years). They also had a higher mean BMI (36.7 kg/m2) and were more likely to be men than those with FIB-4 score ≥1.3. Of those with a baseline FIB-4 measurement outside the guideline-recommended category for moderate-to-advanced fibrosis (n=480), 91.3% had other NITs within the guideline-recommended category (ELF score ≥9.0–< 11.3 or VCTE LSM ≥8.0–< 20.0 kPa) (Table 2). Of note, 27.6% of participants had ≥1 NIT score that was above the guideline-recommended limit, however, only 1.3% had values above the upper limits of all NITs (Table 2). Additionally, those with VCTE LSM scores ≥20.0 kPa had a higher mean BMI (38.5 kg/m2) and were more likely to have type 2 diabetes (61.0%) than those with VCTE LSM < 20.0 kPa (Table 1).
Discussion: While participants in ESSENCE had biopsy-defined stage 2 or 3 fibrosis, the population had a broad range of NIT values. If there is a clinical suspicion for MASH with moderate fibrosis, use of multiple NITs may support a clinical diagnosis of MASH.
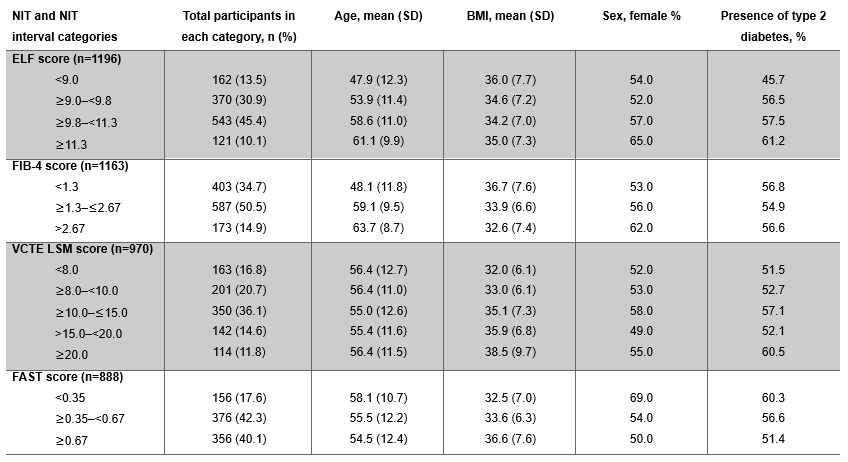
Figure: Table 1. Baseline NIT score groupings in those with a recorded measurement in the ESSENCE randomized population (N=1197). BMI, body mass index; ELF, enhanced liver fibrosis; FAST, FibroScan-AST; FIB-4; Fibrosis-4; NIT, non-invasive test; SD, standard deviation; VCTE LSM, vibration-controlled transient elastography liver stiffness measurement.
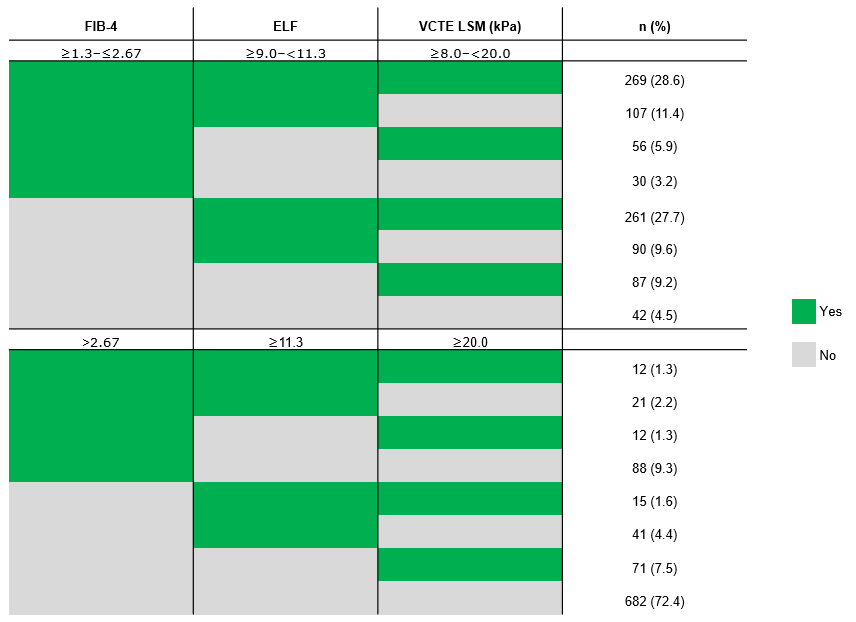
Figure: Table 2. NIT cutoff cross table in those with histologically confirmed fibrosis stage 2 or 3 and with a baseline measurement in reported NITs (N=942). ELF, enhanced liver fibrosis; FIB-4, Fibrosis-4; NIT, non-invasive test; VCTE LSM, vibration-controlled transient elastography liver stiffness measurement.
Disclosures:
Laurent Castera: AstraZeneca – Lecture fees. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Echosens – Consultant, Lecture fees. Gilead – Lecture fees. GSK – Consultant. Inventiva – Lecture fees. Madrigal – Consultant, Lecture fees. MSD – Consultant. Novo Nordisk – Consultant, Lecture fees. Pfizer – Consultant. Sagimet – Consultant. Siemens Healthineers – Consultant.
Kristiane Engebretsen: Novo Nordisk – Employee, Stock-publicly held company(excluding mutual/index funds).
George Goh: Abbott – Speakers Bureau. Boehringer Ingelheim – Advisory Committee/Board Member. Echosens – Speakers Bureau. Merck – Advisory Committee/Board Member. Novo Nordisk – Advisory Committee/Board Member, Speakers Bureau. Roche Diagnostics – Advisory Committee/Board Member.
Elisabetta Bugianesi: Boehringer Ingelheim – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member. MSD – Advisor or Review Panel Member. Novo Nordisk – Advisor or Review Panel Member.
Anna Calí: Novo Nordisk – Employee, Stock-publicly held company(excluding mutual/index funds).
Burak Demirel: Novo Nordisk – Employee, Stock-publicly held company(excluding mutual/index funds).
Tomáš Koller: Abbvie – Travel grant. Gilead Sciences – Travel grant. Johnson & Johnson – Advisory Committee/Board Member. Novo Nordisk – Clinical studies.
Niels Krarup: Novo Nordisk – Employee, Stock-publicly held company(excluding mutual/index funds).
Michelle Long: Novo Nordisk – Employee, Stock-publicly held company(excluding mutual/index funds).
Philip Newsome: AiCME – Speakers Bureau. Boehringer Ingelheim – Advisor or Review Panel Member, Consultant. Bristol Myers Squibb – Advisor or Review Panel Member, Consultant. Gilead – Advisor or Review Panel Member, Consultant. GSK – Advisor or Review Panel Member, Consultant. Intercept – Advisor or Review Panel Member, Consultant. Madrigal – Advisor or Review Panel Member, Consultant. Novo Nordisk – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Consultant. Poxel – Advisor or Review Panel Member, Consultant. Sun Pharma – Advisor or Review Panel Member, Consultant.
Cláudia Oliveira: AstraZeneca – Consultant or participant in clinical trials. Boehringer Ingelheim – Consultant or participant in clinical trials. Inventiva – Consultant or participant in clinical trials. Novo Nordisk – Consultant or participant in clinical trials. Pfizer – Consultant or participant in clinical trials.
Vlad Ratziu: Boehringer Ingelheim – Consultant. Eny – Consultant. Gilead – Grant/Research Support. Intercept – Consultant, Grant/Research Support. Madrigal – Consultant. NorthSea – Consultant. Novo Nordisk – Consultant. Poxel – Consultant. Sagimet – Consultant.
Mary Rinella: Boehringer Ingelheim – Consultant. CytoDyn – Consultant. GSK – Consultant. HistoIndex – Consultant. Intercept – Consultant. Madrigal – Consultant. NGM Bio – Consultant. Novo Nordisk – Consultant. Sonic Incytes – Consultant.
Michael Roden: AstraZeneca – Speaker and/or advisor. Boehringer Ingelheim – Speaker and/or advisor; Center received research grant support for investigator-initiated studies. Echosens – Speaker and/or advisor. Eli Lilly – Speaker and/or advisor. Madrigal – Speaker and/or advisor. MSD – Speaker and/or advisor. Novo Nordisk – Speaker and/or advisor; Center received research grant support for investigator-initiated studies. Synlab – Speaker and/or advisor. TARGET RWE – Speaker and/or advisor.
Giada Sebastiani: AbbVie – Speakers Bureau. Gilead – Advisory Committee/Board Member, Speakers Bureau. Intercept – Advisory Committee/Board Member. Merck – Advisory Committee/Board Member, Speakers Bureau. Novo Nordisk – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Theratec – Grant/Research Support.
Arun Sanyal: 89Bio – Advisory Committee/Board Member, Consultant. Albireo – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. AMRA – Advisory Committee/Board Member, Consultant. ARTham Therapeutics – Consultant. AstraZeneca – Advisory Committee/Board Member, Consultant. Bird Rock Bio – Consultant. Blade Therapeutics – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant, Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Grant/Research Support. Conatus – Advisory Committee/Board Member, Consultant, Grant/Research Support. Covance – Advisory Committee/Board Member, Consultant, Grant/Research Support. Cumberland Pharmaceuticals – Grant/Research Support. CymaBay Therapeutics – Research collaborations. Durect – Stock Options. Echosens – Consultant, Grant/Research Support. Eli Lilly – Advisory Committee/Board Member, Consultant. Exhalenz – Stock Options. Fractyl – Grant/Research Support. Galmed Pharmaceuticals Ltd – Stock Options. Genentech – Advisory Committee/Board Member, Consultant. GENFIT – Advisory Committee/Board Member, Consultant, Stock Options. Gilead – Advisory Committee/Board Member, Consultant, Grant/Research Support. Glympse Bio – Consultant. Hemoshear – Advisory Committee/Board Member, Consultant, Stock Options. HistoIndex – Advisory Committee/Board Member, Consultant. Immuron – Grant/Research Support. Indalo – Stock Options. Intercept Pharmaceuticals – Grant/Research Support. Inventiva – Advisory Committee/Board Member, Consultant, Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Labcorp – Research collaborations. Lilly – Grant/Research Support. Madrigal – Advisory Committee/Board Member, Consultant, Grant/Research Support. Malinckrodt – Advisory Committee/Board Member, Consultant, Grant/Research Support. MedImmune – Advisory Committee/Board Member, Consultant. Merck Sharp & Dohme – Advisory Committee/Board Member, Consultant, Grant/Research Support. NASH Pharmaceuticals – Consultant. NGM Bio – Advisory Committee/Board Member, Consultant. NorthSea – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant, Grant/Research Support. Novo Nordisk – Advisory Committee/Board Member, Consultant, Grant/Research Support. PathAI – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Poxel – Advisory Committee/Board Member, Consultant. ProSciento – Advisory Committee/Board Member, Consultant. Regeneron Pharmaceuticals, Inc – Advisory Committee/Board Member, Consultant. Rivus – Stock Options. Roche – Advisory Committee/Board Member, Consultant. Salix – Advisory Committee/Board Member, Consultant. Sanofi – Advisory Committee/Board Member, Consultant. Sanyal Bio – Stock Options. Second Genome – Research collaborations. Sequana Therapeutics – Grant/Research Support. Siemens – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. Terns – Advisory Committee/Board Member, Consultant. Teva Pharmaceutical Industries Ltd – Consultant. Tiziana – Stock Options.
Laurent Castera, MD, PhD1, Kristiane Engebretsen, MD, PhD2, George B.. Goh, MBBS, MRCP (UK), M Med (Int Med), FAMS (Singapore)3, Elisabetta Bugianesi, MD, PhD4, Anna M.G.. Calí, MD, MSc2, Burak Demirel, MD2, Tomáš Koller, MD, PhD5, Niels Krarup, MSc2, Michelle T.. Long, MD, MSc2, Philip Newsome, MD, PhD6, Cláudia Oliveira, MD, PhD7, Vlad Ratziu, MD, PhD8, Mary E.. Rinella, MD9, Michael Roden, MD, PhD10, Giada Sebastiani, MD11, Arun J.. Sanyal, MD12. P5830 - The Use of Non-Invasive Tests to Characterise the Total Baseline Population in the Phase 3 Essence Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Université Paris Cité, UMR1149 (CRI), INSERM, Paris, Ile-de-France, France; 2Novo Nordisk A/S, Copenhagen, Hovedstaden, Denmark; 3Department of Gastroenterology and Hepatology, Singapore General Hospital, Singapore, Singapore; 4Department of Medical Sciences, University of Turin, Turin, Piemonte, Italy; 5Subdivision of Gastroenterology and Hepatology, 5th Department of Internal Medicine, Comenius University Faculty of Medicine, University Hospital Bratislava, Bratislava, Bratislava, Slovakia; 6Roger Williams Institute of Liver Studies, Faculty of Life Sciences and Medicine, King’s College London and King’s College Hospital, London, England, United Kingdom; 7Departamento de Gastroenterologia, Hospital das Clínicas (LIM07) da Faculdade de Medicina da Universidade de São Paulo, Sao Paulo, Sao Paulo, Brazil; 8Sorbonne Université, Institute for Cardiometabolism and Nutrition, Hospital Pitié Salpêtrière, INSERM UMRS 1138 CRC, Paris, Ile-de-France, France; 9Division of Gastroenterology, Hepatology and Nutrition, University of Chicago, Chicago, IL; 10Department of Endocrinology and Diabetology, Medical Faculty and University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Dusseldorf, Nordrhein-Westfalen, Germany; 11Division of Gastroenterology and Hepatology, McGill University Health Centre, Montreal, PQ, Canada; 12Virginia Commonwealth University; Central Virginia Veterans Healthcare System, Richmond, VA
Introduction: ESSENCE is an ongoing phase 3 trial (NCT04822181) in participants with biopsy-defined, non-cirrhotic metabolic dysfunction-associated steatohepatitis (MASH).In part 1, the primary histological endpoints were met in the first 800 randomised participants after 72 weeks’ treatment with semaglutide 2.4 mg. The aim of this post hoc analysis was to characterise the total randomised, baseline population using selected non-invasive tests (NITs).
Methods: Randomised participants (N=1197) were characterised by NITs at baseline: vibration-controlled transient elastography liver stiffness measurement (VCTE LSM; FibroScan), enhanced liver fibrosis (ELF) score, FibroScan-aspartate aminotransferase (FAST) score and Fibrosis-4 index (FIB-4) score. Eligible participants had histologically defined MASH and liver fibrosis stage 2 or 3. Clinical NIT categories for moderate-to-advanced fibrosis were used to group participants, and descriptive statistics determined numbers of participants in each NIT category and demographics.
Results: Distribution of results is shown in Table 1. At baseline, 95.5% fulfilled NIT scores in ≥1 of the guideline-recommended ranges for FIB-4 (≥1.3–≤2.67), ELF (≥9.0–< 11.3) or VCTE LSM (≥8.0–< 20.0 kPa) (Table 2). Most had a FAST score ≥0.35 (82.4%) and 34.7% had a FIB-4 score < 1.3 (Table 1). As expected, participants with FIB-4 score < 1.3 were younger (mean age: 48.1 years) than those with FIB-4 score ≥1.3–≤2.67 (59.1 years) and >2.67 (63.7 years). They also had a higher mean BMI (36.7 kg/m2) and were more likely to be men than those with FIB-4 score ≥1.3. Of those with a baseline FIB-4 measurement outside the guideline-recommended category for moderate-to-advanced fibrosis (n=480), 91.3% had other NITs within the guideline-recommended category (ELF score ≥9.0–< 11.3 or VCTE LSM ≥8.0–< 20.0 kPa) (Table 2). Of note, 27.6% of participants had ≥1 NIT score that was above the guideline-recommended limit, however, only 1.3% had values above the upper limits of all NITs (Table 2). Additionally, those with VCTE LSM scores ≥20.0 kPa had a higher mean BMI (38.5 kg/m2) and were more likely to have type 2 diabetes (61.0%) than those with VCTE LSM < 20.0 kPa (Table 1).
Discussion: While participants in ESSENCE had biopsy-defined stage 2 or 3 fibrosis, the population had a broad range of NIT values. If there is a clinical suspicion for MASH with moderate fibrosis, use of multiple NITs may support a clinical diagnosis of MASH.

Figure: Table 1. Baseline NIT score groupings in those with a recorded measurement in the ESSENCE randomized population (N=1197). BMI, body mass index; ELF, enhanced liver fibrosis; FAST, FibroScan-AST; FIB-4; Fibrosis-4; NIT, non-invasive test; SD, standard deviation; VCTE LSM, vibration-controlled transient elastography liver stiffness measurement.

Figure: Table 2. NIT cutoff cross table in those with histologically confirmed fibrosis stage 2 or 3 and with a baseline measurement in reported NITs (N=942). ELF, enhanced liver fibrosis; FIB-4, Fibrosis-4; NIT, non-invasive test; VCTE LSM, vibration-controlled transient elastography liver stiffness measurement.
Disclosures:
Laurent Castera: AstraZeneca – Lecture fees. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Echosens – Consultant, Lecture fees. Gilead – Lecture fees. GSK – Consultant. Inventiva – Lecture fees. Madrigal – Consultant, Lecture fees. MSD – Consultant. Novo Nordisk – Consultant, Lecture fees. Pfizer – Consultant. Sagimet – Consultant. Siemens Healthineers – Consultant.
Kristiane Engebretsen: Novo Nordisk – Employee, Stock-publicly held company(excluding mutual/index funds).
George Goh: Abbott – Speakers Bureau. Boehringer Ingelheim – Advisory Committee/Board Member. Echosens – Speakers Bureau. Merck – Advisory Committee/Board Member. Novo Nordisk – Advisory Committee/Board Member, Speakers Bureau. Roche Diagnostics – Advisory Committee/Board Member.
Elisabetta Bugianesi: Boehringer Ingelheim – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member. MSD – Advisor or Review Panel Member. Novo Nordisk – Advisor or Review Panel Member.
Anna Calí: Novo Nordisk – Employee, Stock-publicly held company(excluding mutual/index funds).
Burak Demirel: Novo Nordisk – Employee, Stock-publicly held company(excluding mutual/index funds).
Tomáš Koller: Abbvie – Travel grant. Gilead Sciences – Travel grant. Johnson & Johnson – Advisory Committee/Board Member. Novo Nordisk – Clinical studies.
Niels Krarup: Novo Nordisk – Employee, Stock-publicly held company(excluding mutual/index funds).
Michelle Long: Novo Nordisk – Employee, Stock-publicly held company(excluding mutual/index funds).
Philip Newsome: AiCME – Speakers Bureau. Boehringer Ingelheim – Advisor or Review Panel Member, Consultant. Bristol Myers Squibb – Advisor or Review Panel Member, Consultant. Gilead – Advisor or Review Panel Member, Consultant. GSK – Advisor or Review Panel Member, Consultant. Intercept – Advisor or Review Panel Member, Consultant. Madrigal – Advisor or Review Panel Member, Consultant. Novo Nordisk – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Consultant. Poxel – Advisor or Review Panel Member, Consultant. Sun Pharma – Advisor or Review Panel Member, Consultant.
Cláudia Oliveira: AstraZeneca – Consultant or participant in clinical trials. Boehringer Ingelheim – Consultant or participant in clinical trials. Inventiva – Consultant or participant in clinical trials. Novo Nordisk – Consultant or participant in clinical trials. Pfizer – Consultant or participant in clinical trials.
Vlad Ratziu: Boehringer Ingelheim – Consultant. Eny – Consultant. Gilead – Grant/Research Support. Intercept – Consultant, Grant/Research Support. Madrigal – Consultant. NorthSea – Consultant. Novo Nordisk – Consultant. Poxel – Consultant. Sagimet – Consultant.
Mary Rinella: Boehringer Ingelheim – Consultant. CytoDyn – Consultant. GSK – Consultant. HistoIndex – Consultant. Intercept – Consultant. Madrigal – Consultant. NGM Bio – Consultant. Novo Nordisk – Consultant. Sonic Incytes – Consultant.
Michael Roden: AstraZeneca – Speaker and/or advisor. Boehringer Ingelheim – Speaker and/or advisor; Center received research grant support for investigator-initiated studies. Echosens – Speaker and/or advisor. Eli Lilly – Speaker and/or advisor. Madrigal – Speaker and/or advisor. MSD – Speaker and/or advisor. Novo Nordisk – Speaker and/or advisor; Center received research grant support for investigator-initiated studies. Synlab – Speaker and/or advisor. TARGET RWE – Speaker and/or advisor.
Giada Sebastiani: AbbVie – Speakers Bureau. Gilead – Advisory Committee/Board Member, Speakers Bureau. Intercept – Advisory Committee/Board Member. Merck – Advisory Committee/Board Member, Speakers Bureau. Novo Nordisk – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Theratec – Grant/Research Support.
Arun Sanyal: 89Bio – Advisory Committee/Board Member, Consultant. Albireo – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. AMRA – Advisory Committee/Board Member, Consultant. ARTham Therapeutics – Consultant. AstraZeneca – Advisory Committee/Board Member, Consultant. Bird Rock Bio – Consultant. Blade Therapeutics – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant, Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Grant/Research Support. Conatus – Advisory Committee/Board Member, Consultant, Grant/Research Support. Covance – Advisory Committee/Board Member, Consultant, Grant/Research Support. Cumberland Pharmaceuticals – Grant/Research Support. CymaBay Therapeutics – Research collaborations. Durect – Stock Options. Echosens – Consultant, Grant/Research Support. Eli Lilly – Advisory Committee/Board Member, Consultant. Exhalenz – Stock Options. Fractyl – Grant/Research Support. Galmed Pharmaceuticals Ltd – Stock Options. Genentech – Advisory Committee/Board Member, Consultant. GENFIT – Advisory Committee/Board Member, Consultant, Stock Options. Gilead – Advisory Committee/Board Member, Consultant, Grant/Research Support. Glympse Bio – Consultant. Hemoshear – Advisory Committee/Board Member, Consultant, Stock Options. HistoIndex – Advisory Committee/Board Member, Consultant. Immuron – Grant/Research Support. Indalo – Stock Options. Intercept Pharmaceuticals – Grant/Research Support. Inventiva – Advisory Committee/Board Member, Consultant, Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Labcorp – Research collaborations. Lilly – Grant/Research Support. Madrigal – Advisory Committee/Board Member, Consultant, Grant/Research Support. Malinckrodt – Advisory Committee/Board Member, Consultant, Grant/Research Support. MedImmune – Advisory Committee/Board Member, Consultant. Merck Sharp & Dohme – Advisory Committee/Board Member, Consultant, Grant/Research Support. NASH Pharmaceuticals – Consultant. NGM Bio – Advisory Committee/Board Member, Consultant. NorthSea – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant, Grant/Research Support. Novo Nordisk – Advisory Committee/Board Member, Consultant, Grant/Research Support. PathAI – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Poxel – Advisory Committee/Board Member, Consultant. ProSciento – Advisory Committee/Board Member, Consultant. Regeneron Pharmaceuticals, Inc – Advisory Committee/Board Member, Consultant. Rivus – Stock Options. Roche – Advisory Committee/Board Member, Consultant. Salix – Advisory Committee/Board Member, Consultant. Sanofi – Advisory Committee/Board Member, Consultant. Sanyal Bio – Stock Options. Second Genome – Research collaborations. Sequana Therapeutics – Grant/Research Support. Siemens – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. Terns – Advisory Committee/Board Member, Consultant. Teva Pharmaceutical Industries Ltd – Consultant. Tiziana – Stock Options.
Laurent Castera, MD, PhD1, Kristiane Engebretsen, MD, PhD2, George B.. Goh, MBBS, MRCP (UK), M Med (Int Med), FAMS (Singapore)3, Elisabetta Bugianesi, MD, PhD4, Anna M.G.. Calí, MD, MSc2, Burak Demirel, MD2, Tomáš Koller, MD, PhD5, Niels Krarup, MSc2, Michelle T.. Long, MD, MSc2, Philip Newsome, MD, PhD6, Cláudia Oliveira, MD, PhD7, Vlad Ratziu, MD, PhD8, Mary E.. Rinella, MD9, Michael Roden, MD, PhD10, Giada Sebastiani, MD11, Arun J.. Sanyal, MD12. P5830 - The Use of Non-Invasive Tests to Characterise the Total Baseline Population in the Phase 3 Essence Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
