Tuesday Poster Session
Category: Liver
P5781 - GLP-1 Receptor Agonists in Metabolic Dysfunction-Associated Steatohepatitis: A Meta-Analysis of Efficacy and Safety
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Mazhar Shapoo, MD
Detroit Medical Center/Wayne State University
Detroit, MI
Presenting Author(s)
Mazhar Shapoo, MD1, Anas Babar, MBBS2, Hafiz Muhammad Ehsan Arshad, MBBS2, Faaz Ali, MBBS2, Hafiz Hamdoon Saleem, MBBS2, Omar Abdullah Gill, MBBS2, Muhammad Zain Raza, MBBS2, Musab Maqsood, MBBS2, Muhammad Omais, MBBS2, Muhammad Abdul Rehman, MBBS3
1Detroit Medical Center/Wayne State University, Detroit, MI; 2King Edward Medical University, Lahore, Punjab, Pakistan; 3Mayo Clinic, Byron, MN
Introduction: Metabolic dysfunction-associated steatohepatitis (MASH), a severe form of metabolic dysfunction-associated fatty liver disease (MAFLD), is linked to a high risk of liver-related complications as well as all-cause morbidity and mortality. Despite the advent of multiple therapeutic targets currently in late phase of drug development, there is limited consensus regarding the efficacy of these regimens. Given the limited high-quality evidence for first-line regimens, this meta-analysis aims to investigate the efficacy and safety of the glucagon-like peptide-1 analogues (semaglutide and liraglutide) for the treatment of MASH.
Methods: PubMed, Embase, Cochrane, ClinicalTrials.gov, and WHO ICTRP were searched for randomized controlled-trials (RCTs). Outcomes were analyzed using a random-effects model. For dichotomous outcomes Mantel-Haenszel method, and for contrast-level data inverse variance method was used. Heterogeneity was assessed by DerSimonian and Laird, and confidence intervals by the Wald method. All analyses were conducted using RevMan Web
Results: Five RCTs consisting of 1,264 patients with confirmed MASH were included. Compared with placebo, treatment with semaglutide and liraglutide in a significantly higher rate of MASH resolution (OR:3.29; 95%-CI:2.50, 4.32; P< 0.00001) and demonstrated a pooled mean reduction in liver stiffness of −1.86 kPa (95%-CI: -3.43, -0.30). However, no significant difference was observed in the proportion of patients achieving a reduction in liver fibrosis stage (OR:1.43; 95%-CI:0.75, 2.74; P=0.28). While treatment was associated with a significantly increased risk of gastrointestinal adverse events (OR:3.51; 95%-CI:2.70, 4.56; P< 0.00001), there was no statistically significant difference in the incidence of serious adverse events (OR:1.10; 95%-CI:0.81, 1.51; P=0.54) or treatment discontinuations (OR:1.01; 95%-CI:0.58, 1.77; P=0.97).
Discussion: GLP-1 receptor agonists, particularly semaglutide, significantly improve liver histology in patients with MASH, especially among those with moderate to advanced fibrosis. The overall favorable safety profile, aside from an increased incidence of gastrointestinal adverse events, supports the potential role of these agents as first-line therapy in MASH management. Nevertheless, studies with broader population groups and longer duration are needed to determine the long-term impact of gastrointestinal side effects on treatment adherence and patient quality of life
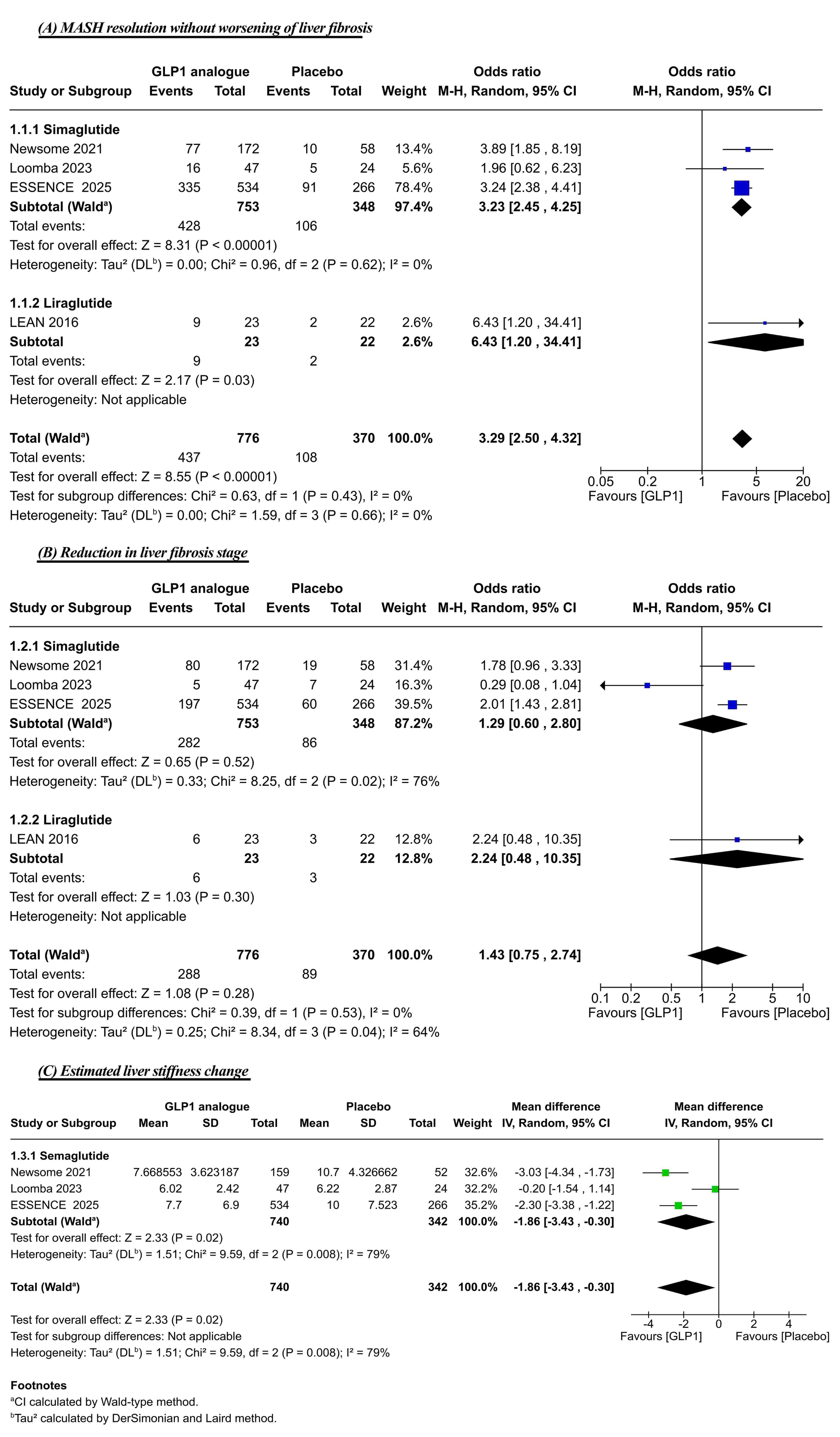
Figure: Figure 1: Table summarizing the findings of the meta-analysis.
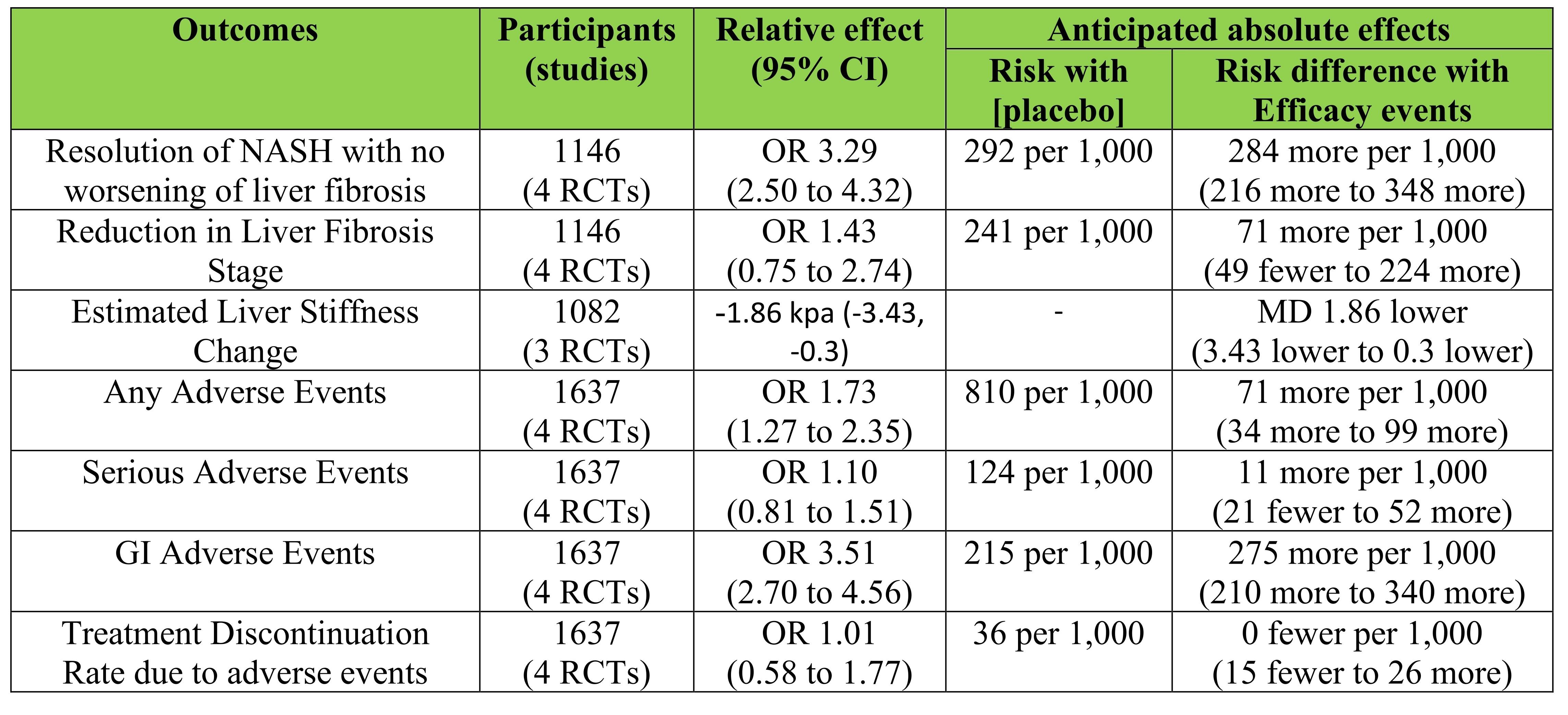
Figure: Figure 2: Forest plots of (A) MASH resolution without worsening of liver fibrosis, (B) Reduction in liver fibrosis stage, and (C) Estimated liver stiffness change.
Disclosures:
Mazhar Shapoo indicated no relevant financial relationships.
Anas Babar indicated no relevant financial relationships.
Hafiz Muhammad Ehsan Arshad indicated no relevant financial relationships.
Faaz Ali indicated no relevant financial relationships.
Hafiz Hamdoon Saleem indicated no relevant financial relationships.
Omar Abdullah Gill indicated no relevant financial relationships.
Muhammad Zain Raza indicated no relevant financial relationships.
Musab Maqsood indicated no relevant financial relationships.
Muhammad Omais indicated no relevant financial relationships.
Muhammad Abdul Rehman indicated no relevant financial relationships.
Mazhar Shapoo, MD1, Anas Babar, MBBS2, Hafiz Muhammad Ehsan Arshad, MBBS2, Faaz Ali, MBBS2, Hafiz Hamdoon Saleem, MBBS2, Omar Abdullah Gill, MBBS2, Muhammad Zain Raza, MBBS2, Musab Maqsood, MBBS2, Muhammad Omais, MBBS2, Muhammad Abdul Rehman, MBBS3. P5781 - GLP-1 Receptor Agonists in Metabolic Dysfunction-Associated Steatohepatitis: A Meta-Analysis of Efficacy and Safety, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Detroit Medical Center/Wayne State University, Detroit, MI; 2King Edward Medical University, Lahore, Punjab, Pakistan; 3Mayo Clinic, Byron, MN
Introduction: Metabolic dysfunction-associated steatohepatitis (MASH), a severe form of metabolic dysfunction-associated fatty liver disease (MAFLD), is linked to a high risk of liver-related complications as well as all-cause morbidity and mortality. Despite the advent of multiple therapeutic targets currently in late phase of drug development, there is limited consensus regarding the efficacy of these regimens. Given the limited high-quality evidence for first-line regimens, this meta-analysis aims to investigate the efficacy and safety of the glucagon-like peptide-1 analogues (semaglutide and liraglutide) for the treatment of MASH.
Methods: PubMed, Embase, Cochrane, ClinicalTrials.gov, and WHO ICTRP were searched for randomized controlled-trials (RCTs). Outcomes were analyzed using a random-effects model. For dichotomous outcomes Mantel-Haenszel method, and for contrast-level data inverse variance method was used. Heterogeneity was assessed by DerSimonian and Laird, and confidence intervals by the Wald method. All analyses were conducted using RevMan Web
Results: Five RCTs consisting of 1,264 patients with confirmed MASH were included. Compared with placebo, treatment with semaglutide and liraglutide in a significantly higher rate of MASH resolution (OR:3.29; 95%-CI:2.50, 4.32; P< 0.00001) and demonstrated a pooled mean reduction in liver stiffness of −1.86 kPa (95%-CI: -3.43, -0.30). However, no significant difference was observed in the proportion of patients achieving a reduction in liver fibrosis stage (OR:1.43; 95%-CI:0.75, 2.74; P=0.28). While treatment was associated with a significantly increased risk of gastrointestinal adverse events (OR:3.51; 95%-CI:2.70, 4.56; P< 0.00001), there was no statistically significant difference in the incidence of serious adverse events (OR:1.10; 95%-CI:0.81, 1.51; P=0.54) or treatment discontinuations (OR:1.01; 95%-CI:0.58, 1.77; P=0.97).
Discussion: GLP-1 receptor agonists, particularly semaglutide, significantly improve liver histology in patients with MASH, especially among those with moderate to advanced fibrosis. The overall favorable safety profile, aside from an increased incidence of gastrointestinal adverse events, supports the potential role of these agents as first-line therapy in MASH management. Nevertheless, studies with broader population groups and longer duration are needed to determine the long-term impact of gastrointestinal side effects on treatment adherence and patient quality of life

Figure: Figure 1: Table summarizing the findings of the meta-analysis.

Figure: Figure 2: Forest plots of (A) MASH resolution without worsening of liver fibrosis, (B) Reduction in liver fibrosis stage, and (C) Estimated liver stiffness change.
Disclosures:
Mazhar Shapoo indicated no relevant financial relationships.
Anas Babar indicated no relevant financial relationships.
Hafiz Muhammad Ehsan Arshad indicated no relevant financial relationships.
Faaz Ali indicated no relevant financial relationships.
Hafiz Hamdoon Saleem indicated no relevant financial relationships.
Omar Abdullah Gill indicated no relevant financial relationships.
Muhammad Zain Raza indicated no relevant financial relationships.
Musab Maqsood indicated no relevant financial relationships.
Muhammad Omais indicated no relevant financial relationships.
Muhammad Abdul Rehman indicated no relevant financial relationships.
Mazhar Shapoo, MD1, Anas Babar, MBBS2, Hafiz Muhammad Ehsan Arshad, MBBS2, Faaz Ali, MBBS2, Hafiz Hamdoon Saleem, MBBS2, Omar Abdullah Gill, MBBS2, Muhammad Zain Raza, MBBS2, Musab Maqsood, MBBS2, Muhammad Omais, MBBS2, Muhammad Abdul Rehman, MBBS3. P5781 - GLP-1 Receptor Agonists in Metabolic Dysfunction-Associated Steatohepatitis: A Meta-Analysis of Efficacy and Safety, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
