Tuesday Poster Session
Category: Interventional Endoscopy
P5700 - Expanding the Role of Lumen-Apposing Metal Stents: Off-Label Applications in Gastrointestinal Disease
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Blake Flores Semyonov, DO
HCA Florida Largo Hospital
Nashville, TN
Presenting Author(s)
Award: ACG Presidential Poster Award
Blake Flores Semyonov, DO1, Meir Mizrahi, MD2
1HCA Florida Largo Hospital, Pinellas Park, FL; 2HCA Florida Largo Hospital, Largo, FL
Introduction: Lumen-apposing metal stents (LAMS) are FDA-approved for the drainage of pancreatic pseudocysts, walled-off necrosis, and gallbladder drainage in high-risk surgical candidates. In clinical practice, LAMS are used off-label in a range of gastrointestinal conditions, including enteral anastomosis, access to excluded anatomy, and management of benign strictures. This case series highlights three off-label applications demonstrating the versatility, effectiveness, and safety of LAMS.
Case Description/
Methods: In the first case, a patient with a history of Roux-en-Y gastric bypass presented with abdominal pain, nausea, and vomiting. She was unable to tolerate any oral intake and required total parenteral nutrition. Endoscopy revealed a gastrojejunal anastomotic stricture. A 20 mm LAMS was deployed across the stricture, resulting in resolution of symptoms and return to oral feeding.
In the second case, a patient with prior Roux-en-Y bypass presented with abdominal pain and elevated liver-associated enzymes. Imaging demonstrated intra- and extrahepatic biliary dilation, choledocholithiasis, and ampullary stenosis. Initial ERCP failed due to altered anatomy. A LAMS was placed via a jejuno-gastric EDGE approach to access the excluded stomach. The stent was dilated to 20 mm and the patient was placed in prone position to permit duodenoscope passage. Balloon dilation and stone extraction were performed with resolution of biliary obstruction.
In the third case, a patient with pancreatic cancer and hepatic metastases presented with abdominal pain, nausea, and vomiting. He had a history of biliary stenting for obstructive cholestasis and was being evaluated for chemotherapy. CT showed a distended stomach with an abrupt transition at the second portion of the duodenum. An EUS-guided gastrojejunostomy was performed using a 20 mm LAMS with rapid symptomatic improvement.
Discussion: These cases demonstrate the expanding role of LAMS in therapeutic endoscopy, particularly in off-label settings. While their approved indications remain limited to pancreatic fluid collections and cholecystostomy in select patients, real-world use has extended into areas such as stricture management, transgastric ERCP, and enteral bypass for malignant obstruction. With increasing experience and published outcomes, the need for prospective trials and regulatory consideration is clear. These would help establish safety, standardize technique, and expand access to these innovative therapies.
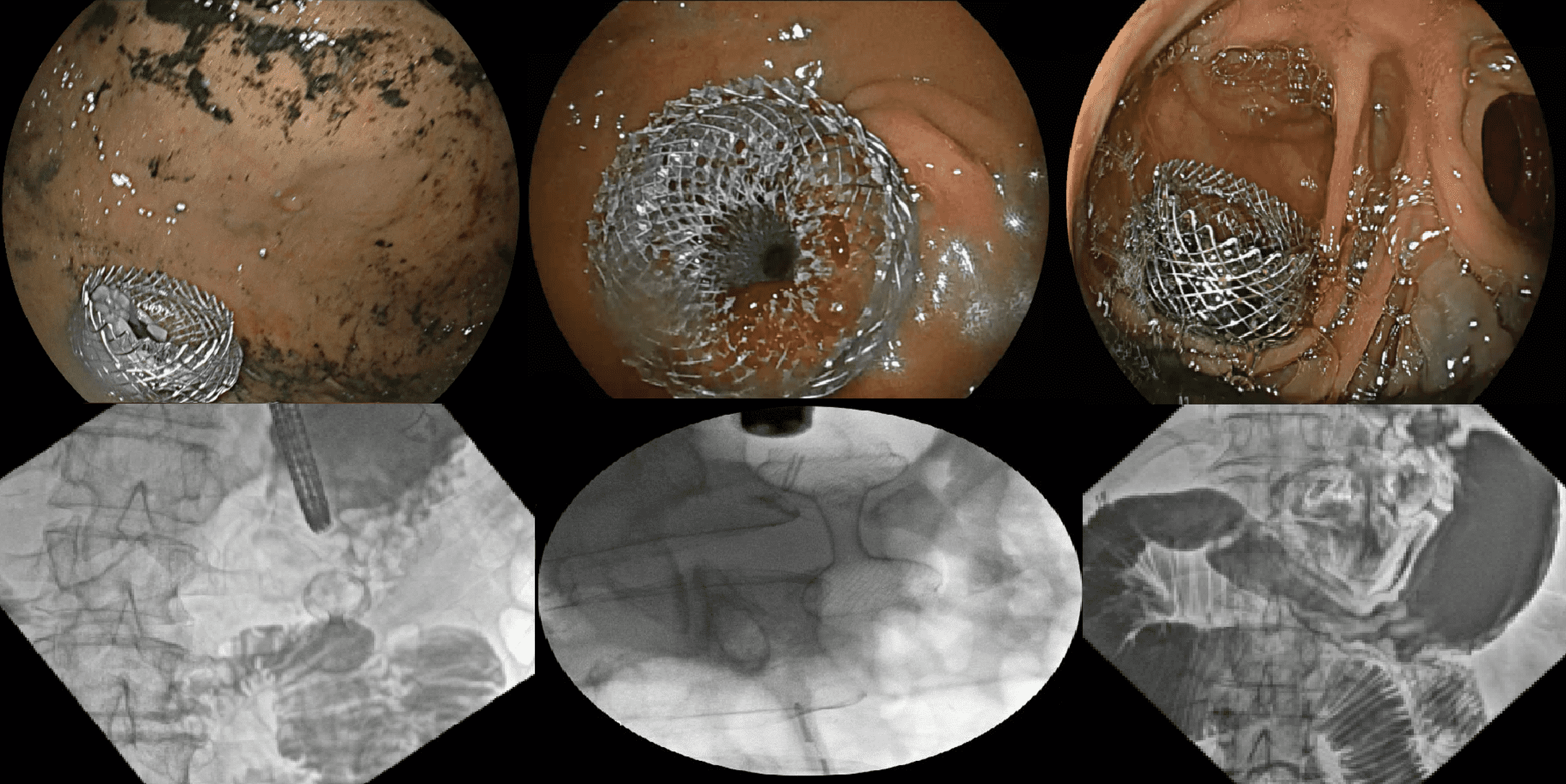
Figure: Three off-label applications of LAMS in gastrointestinal endoscopy: enteral anastomosis, access to excluded anatomy, and management of benign strictures.
Disclosures:
Blake Flores Semyonov indicated no relevant financial relationships.
Meir Mizrahi: Boston Scientific – Consultant. FUJIFILM – Consultant. Medtronic – Consultant.
Blake Flores Semyonov, DO1, Meir Mizrahi, MD2. P5700 - Expanding the Role of Lumen-Apposing Metal Stents: Off-Label Applications in Gastrointestinal Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Blake Flores Semyonov, DO1, Meir Mizrahi, MD2
1HCA Florida Largo Hospital, Pinellas Park, FL; 2HCA Florida Largo Hospital, Largo, FL
Introduction: Lumen-apposing metal stents (LAMS) are FDA-approved for the drainage of pancreatic pseudocysts, walled-off necrosis, and gallbladder drainage in high-risk surgical candidates. In clinical practice, LAMS are used off-label in a range of gastrointestinal conditions, including enteral anastomosis, access to excluded anatomy, and management of benign strictures. This case series highlights three off-label applications demonstrating the versatility, effectiveness, and safety of LAMS.
Case Description/
Methods: In the first case, a patient with a history of Roux-en-Y gastric bypass presented with abdominal pain, nausea, and vomiting. She was unable to tolerate any oral intake and required total parenteral nutrition. Endoscopy revealed a gastrojejunal anastomotic stricture. A 20 mm LAMS was deployed across the stricture, resulting in resolution of symptoms and return to oral feeding.
In the second case, a patient with prior Roux-en-Y bypass presented with abdominal pain and elevated liver-associated enzymes. Imaging demonstrated intra- and extrahepatic biliary dilation, choledocholithiasis, and ampullary stenosis. Initial ERCP failed due to altered anatomy. A LAMS was placed via a jejuno-gastric EDGE approach to access the excluded stomach. The stent was dilated to 20 mm and the patient was placed in prone position to permit duodenoscope passage. Balloon dilation and stone extraction were performed with resolution of biliary obstruction.
In the third case, a patient with pancreatic cancer and hepatic metastases presented with abdominal pain, nausea, and vomiting. He had a history of biliary stenting for obstructive cholestasis and was being evaluated for chemotherapy. CT showed a distended stomach with an abrupt transition at the second portion of the duodenum. An EUS-guided gastrojejunostomy was performed using a 20 mm LAMS with rapid symptomatic improvement.
Discussion: These cases demonstrate the expanding role of LAMS in therapeutic endoscopy, particularly in off-label settings. While their approved indications remain limited to pancreatic fluid collections and cholecystostomy in select patients, real-world use has extended into areas such as stricture management, transgastric ERCP, and enteral bypass for malignant obstruction. With increasing experience and published outcomes, the need for prospective trials and regulatory consideration is clear. These would help establish safety, standardize technique, and expand access to these innovative therapies.

Figure: Three off-label applications of LAMS in gastrointestinal endoscopy: enteral anastomosis, access to excluded anatomy, and management of benign strictures.
Disclosures:
Blake Flores Semyonov indicated no relevant financial relationships.
Meir Mizrahi: Boston Scientific – Consultant. FUJIFILM – Consultant. Medtronic – Consultant.
Blake Flores Semyonov, DO1, Meir Mizrahi, MD2. P5700 - Expanding the Role of Lumen-Apposing Metal Stents: Off-Label Applications in Gastrointestinal Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

