Tuesday Poster Session
Category: Interventional Endoscopy
P5652 - Adverse Events Associated With Full-Thickness Resection Devices in Gastrointestinal Endoscopy: Analysis of the FDA MAUDE Database
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Muhammad Shahzil, MD
Penn State Health Milton S. Hershey Medical Center
Hershey, PA
Presenting Author(s)
Muhammad Shahzil, MD1, Talha Kashif, MBBS2, Ali Akram Qureshi, MBBS2, Minahel Shehzadi, MBBS2, Ikponmwosa Enofe, MD1, Hadie Razjouyan, MD1
1Penn State Health Milton S. Hershey Medical Center, Hershey, PA; 2King Edward Medical University, Lahore, Punjab, Pakistan
Introduction: Endoscopic full-thickness resection (EFTR) using full-thickness resection devices (FTRDs) offers a minimally invasive approach for complex gastrointestinal (GI) lesions, especially those involving deeper layers like the muscularis propria. Despite growing adoption, limited comprehensive safety data restrict the development of procedural guidelines and broader clinical acceptance. Understanding the safety profile and efficacy of EFTR is crucial for optimizing patient outcomes and establishing best practices.
Methods: We conducted a retrospective analysis using the FDA Manufacturer and User Facility Device Experience (MAUDE) database from January 2014 to December 2025. Reports involving the FTRD were reviewed to classify device malfunctions and patient-related adverse events. Statistical analysis utilized SPSS, and results were reported as frequencies.
Results: Sixty-eight FTRD cases were reviewed, with colonic FTRD used in 79.4% of cases, and gastroduodenal and diagnostic sets each at 10.3%. Among device-related issues (n=69), clip non-deployment was most prevalent (79.7%). Snare malfunctions (breakage, slippage, resection failure) accounted for 10.1%, and clip detachment occurred in 5.8%. Grasper malfunction (2.9%), improper clip placement, and thread rupture (each 1.5%) were less frequent. Patient adverse events (n=77) were mainly colonic perforations (69.5%), with delayed gastric and colonic perforations each at 3.9%. Duodenal perforations, hemorrhage, and esophageal perforations with mediastinitis occurred in 2.6% each. Four fatalities (5.2%) occurred, two from unrecognized esophageal perforation causing mediastinitis and sepsis, and two post-surgery in patients with significant comorbidities. Surgical intervention was required in 78.7% of complications, with endoscopic clipping alone successful in 3.3% and combined endoscopic/OTSC clipping with surgery in 16.4%.
Discussion: Device maldeployment and perforation are among the two most significant adverse events associated with the use of the FTRD. Although these complications are relatively rare, they can lead to serious clinical consequences. To minimize these risks, comprehensive initial training is essential, along with periodic refresher training—particularly in low-volume centers. Additionally, careful patient selection and ongoing improvements in device engineering are crucial to further enhance the safety and efficacy of this valuable therapeutic tool.
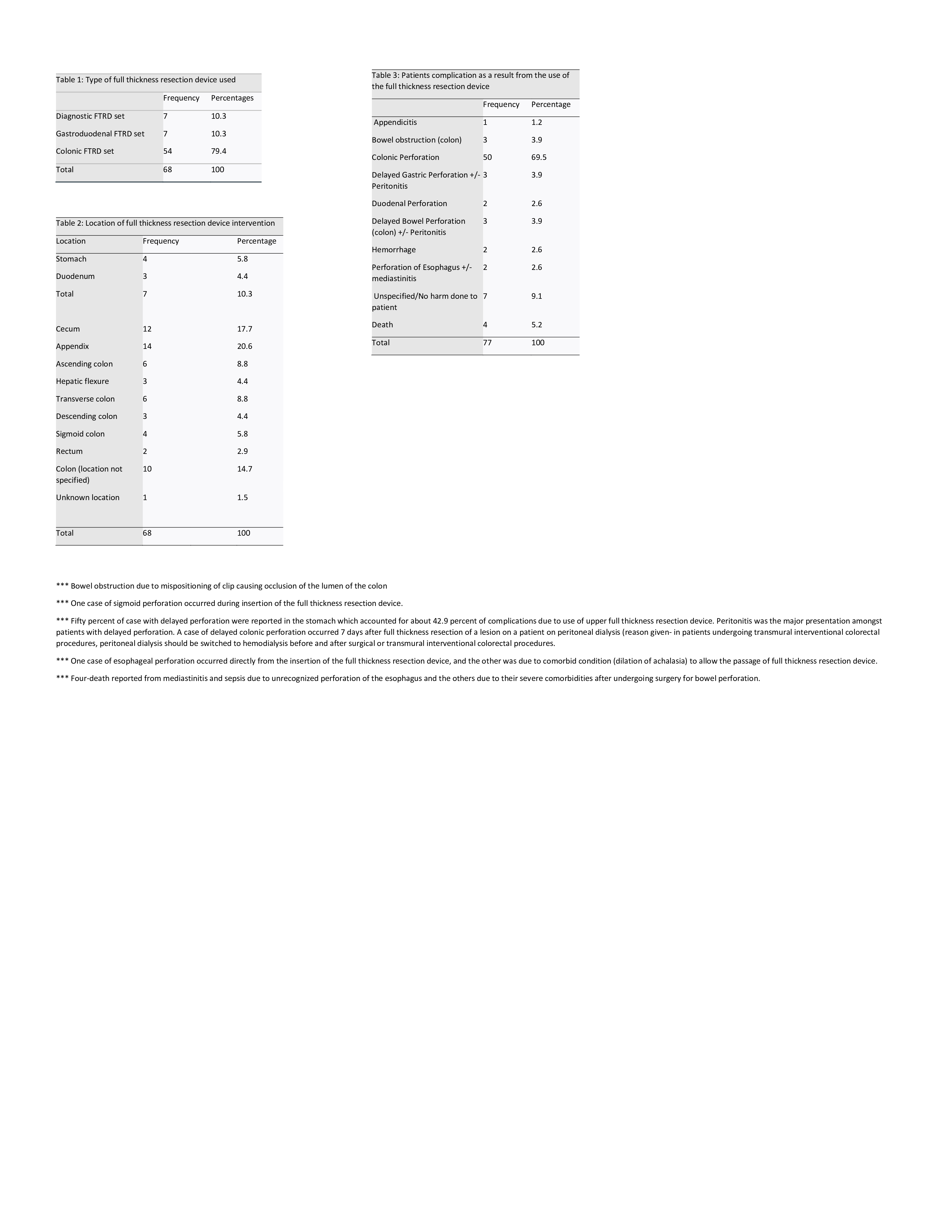
Figure: Device Utilization, Procedure Locations, and Patient Complications
(A) Distribution of full-thickness resection device (FTRD) types used in reported cases from the FDA MAUDE database, with colonic FTRD comprising the majority (79.4%).
(B) Anatomical locations targeted during FTRD deployment, with the appendix (20.6%) and cecum (17.7%) being the most common.
(C) Frequency and types of patient-related complications following FTRD use, where colonic perforation was the most prevalent adverse event (69.5%).
Note: Detailed footnotes describe unique cases such as delayed perforation in peritoneal dialysis and deaths due to mediastinitis following esophageal perforation.
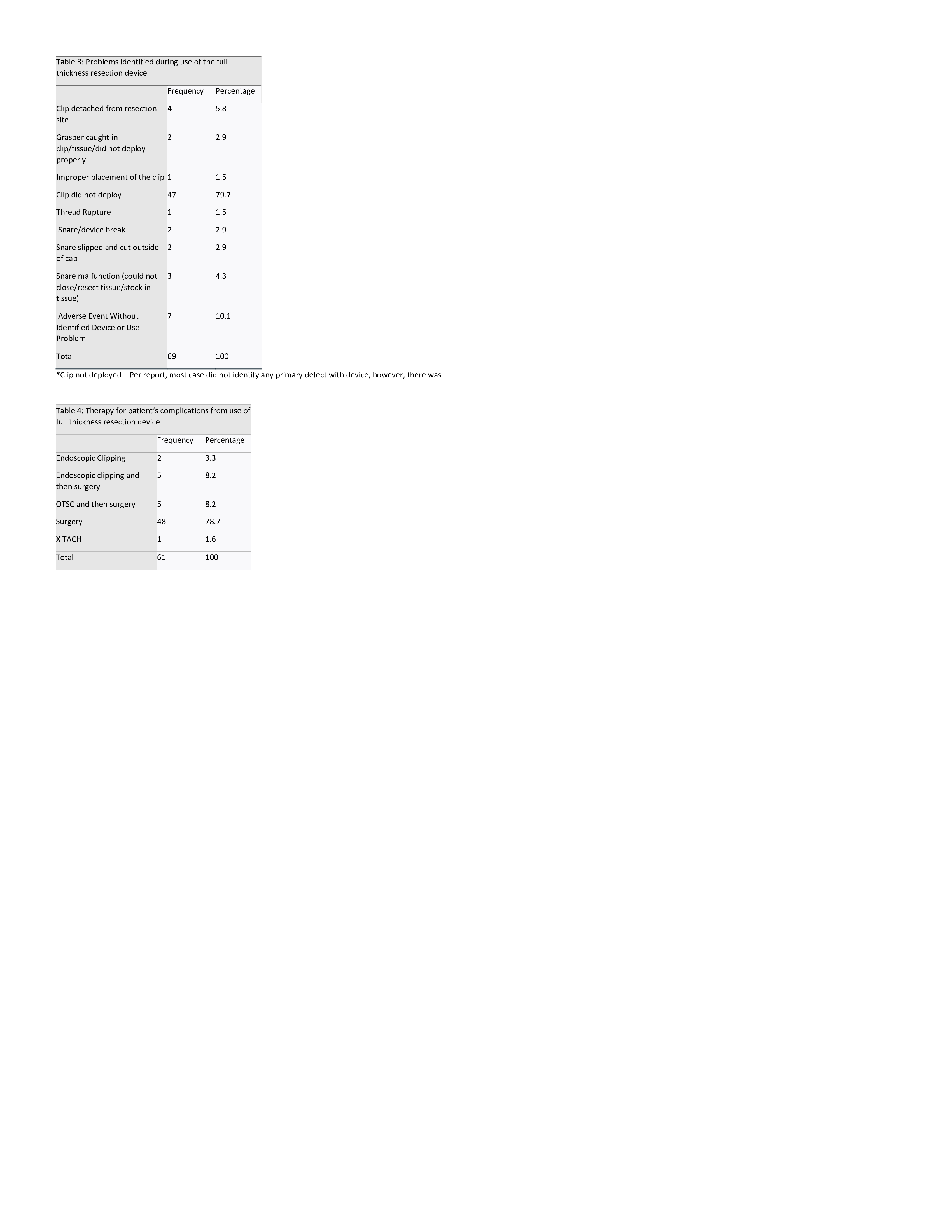
Figure: Device Malfunctions and Therapeutic Interventions
(A) Summary of device-related problems encountered during FTRD use, with clip non-deployment being the predominant failure (79.7%), followed by snare and grasper-related issues.
(B) Therapeutic strategies implemented to address adverse events, where surgical intervention was required in 78.7% of cases. Endoscopic clipping alone was rarely sufficient, while hybrid approaches combining clipping with surgery accounted for 16.4% of management decisions.
Disclosures:
Muhammad Shahzil indicated no relevant financial relationships.
Talha Kashif indicated no relevant financial relationships.
Ali Akram Qureshi indicated no relevant financial relationships.
Minahel Shehzadi indicated no relevant financial relationships.
Ikponmwosa Enofe indicated no relevant financial relationships.
Hadie Razjouyan indicated no relevant financial relationships.
Muhammad Shahzil, MD1, Talha Kashif, MBBS2, Ali Akram Qureshi, MBBS2, Minahel Shehzadi, MBBS2, Ikponmwosa Enofe, MD1, Hadie Razjouyan, MD1. P5652 - Adverse Events Associated With Full-Thickness Resection Devices in Gastrointestinal Endoscopy: Analysis of the FDA MAUDE Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Penn State Health Milton S. Hershey Medical Center, Hershey, PA; 2King Edward Medical University, Lahore, Punjab, Pakistan
Introduction: Endoscopic full-thickness resection (EFTR) using full-thickness resection devices (FTRDs) offers a minimally invasive approach for complex gastrointestinal (GI) lesions, especially those involving deeper layers like the muscularis propria. Despite growing adoption, limited comprehensive safety data restrict the development of procedural guidelines and broader clinical acceptance. Understanding the safety profile and efficacy of EFTR is crucial for optimizing patient outcomes and establishing best practices.
Methods: We conducted a retrospective analysis using the FDA Manufacturer and User Facility Device Experience (MAUDE) database from January 2014 to December 2025. Reports involving the FTRD were reviewed to classify device malfunctions and patient-related adverse events. Statistical analysis utilized SPSS, and results were reported as frequencies.
Results: Sixty-eight FTRD cases were reviewed, with colonic FTRD used in 79.4% of cases, and gastroduodenal and diagnostic sets each at 10.3%. Among device-related issues (n=69), clip non-deployment was most prevalent (79.7%). Snare malfunctions (breakage, slippage, resection failure) accounted for 10.1%, and clip detachment occurred in 5.8%. Grasper malfunction (2.9%), improper clip placement, and thread rupture (each 1.5%) were less frequent. Patient adverse events (n=77) were mainly colonic perforations (69.5%), with delayed gastric and colonic perforations each at 3.9%. Duodenal perforations, hemorrhage, and esophageal perforations with mediastinitis occurred in 2.6% each. Four fatalities (5.2%) occurred, two from unrecognized esophageal perforation causing mediastinitis and sepsis, and two post-surgery in patients with significant comorbidities. Surgical intervention was required in 78.7% of complications, with endoscopic clipping alone successful in 3.3% and combined endoscopic/OTSC clipping with surgery in 16.4%.
Discussion: Device maldeployment and perforation are among the two most significant adverse events associated with the use of the FTRD. Although these complications are relatively rare, they can lead to serious clinical consequences. To minimize these risks, comprehensive initial training is essential, along with periodic refresher training—particularly in low-volume centers. Additionally, careful patient selection and ongoing improvements in device engineering are crucial to further enhance the safety and efficacy of this valuable therapeutic tool.

Figure: Device Utilization, Procedure Locations, and Patient Complications
(A) Distribution of full-thickness resection device (FTRD) types used in reported cases from the FDA MAUDE database, with colonic FTRD comprising the majority (79.4%).
(B) Anatomical locations targeted during FTRD deployment, with the appendix (20.6%) and cecum (17.7%) being the most common.
(C) Frequency and types of patient-related complications following FTRD use, where colonic perforation was the most prevalent adverse event (69.5%).
Note: Detailed footnotes describe unique cases such as delayed perforation in peritoneal dialysis and deaths due to mediastinitis following esophageal perforation.

Figure: Device Malfunctions and Therapeutic Interventions
(A) Summary of device-related problems encountered during FTRD use, with clip non-deployment being the predominant failure (79.7%), followed by snare and grasper-related issues.
(B) Therapeutic strategies implemented to address adverse events, where surgical intervention was required in 78.7% of cases. Endoscopic clipping alone was rarely sufficient, while hybrid approaches combining clipping with surgery accounted for 16.4% of management decisions.
Disclosures:
Muhammad Shahzil indicated no relevant financial relationships.
Talha Kashif indicated no relevant financial relationships.
Ali Akram Qureshi indicated no relevant financial relationships.
Minahel Shehzadi indicated no relevant financial relationships.
Ikponmwosa Enofe indicated no relevant financial relationships.
Hadie Razjouyan indicated no relevant financial relationships.
Muhammad Shahzil, MD1, Talha Kashif, MBBS2, Ali Akram Qureshi, MBBS2, Minahel Shehzadi, MBBS2, Ikponmwosa Enofe, MD1, Hadie Razjouyan, MD1. P5652 - Adverse Events Associated With Full-Thickness Resection Devices in Gastrointestinal Endoscopy: Analysis of the FDA MAUDE Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
